TO: Administrative File: CAG-00439N
FROM: Tamara Syrek Jensen, JD
Director, Coverage and Analysis Group
Joseph Chin, MD, MS
Acting Deputy Director, Coverage and Analysis Group
Lead Medical Officer
Lori Ashby
Acting Director, Division of Medical and Surgical Services
Jamie Hermansen, MPP
Lead Health Policy Analyst
Joseph Dolph Hutter, MD, MA
Medical Officer
SUBJECT: Final National Coverage Determination on Screening for Lung Cancer with Low Dose Computed Tomography (LDCT)
DATE: February 5, 2015
I. Decision
The Centers for Medicare & Medicaid Services (CMS) has determined that the evidence is sufficient to add a lung cancer screening counseling and shared decision making visit, and for appropriate beneficiaries, annual screening for lung cancer with low dose computed tomography (LDCT), as an additional preventive service benefit under the Medicare program only if all of the following criteria are met:
Beneficiary eligibility criteria:
- Age 55 – 77 years;
- Asymptomatic (no signs or symptoms of lung cancer);
- Tobacco smoking history of at least 30 pack-years (one pack-year = smoking one pack per day for one year; 1 pack = 20 cigarettes);
- Current smoker or one who has quit smoking within the last 15 years; and
- Receives a written order for LDCT lung cancer screening that meets the following criteria:
- For the initial LDCT lung cancer screening service: a beneficiary must receive a written order for LDCT lung cancer screening during a lung cancer screening counseling and shared decision making visit, furnished by a physician (as defined in Section 1861(r)(1) of the Social Security Act) or qualified non-physician practitioner (meaning a physician assistant, nurse practitioner, or clinical nurse specialist as defined in §1861(aa)(5) of the Social Security Act). A lung cancer screening counseling and shared decision making visit includes the following elements (and is appropriately documented in the beneficiary’s medical records):
- Determination of beneficiary eligibility including age, absence of signs or symptoms of lung cancer, a specific calculation of cigarette smoking pack-years; and if a former smoker, the number of years since quitting;
- Shared decision making, including the use of one or more decision aids, to include benefits and harms of screening, follow-up diagnostic testing, over-diagnosis, false positive rate, and total radiation exposure;
- Counseling on the importance of adherence to annual lung cancer LDCT screening, impact of comorbidities and ability or willingness to undergo diagnosis and treatment;
- Counseling on the importance of maintaining cigarette smoking abstinence if former smoker; or the importance of smoking cessation if current smoker and, if appropriate, furnishing of information about tobacco cessation interventions; and
- If appropriate, the furnishing of a written order for lung cancer screening with LDCT.
- For subsequent LDCT lung cancer screenings: the beneficiary must receive a written order for LDCT lung cancer screening, which may be furnished during any appropriate visit with a physician (as defined in Section 1861(r)(1) of the Social Security Act) or qualified non-physician practitioner (meaning a physician assistant, nurse practitioner, or clinical nurse specialist as defined in Section 1861(aa)(5) of the Social Security Act). If a physician or qualified non-physician practitioner elects to provide a lung cancer screening counseling and shared decision making visit for subsequent lung cancer screenings with LDCT, the visit must meet the criteria described above for a counseling and shared decision making visit.
-
Written orders for both initial and subsequent LDCT lung cancer screenings must contain the following information, which must also be appropriately documented in the beneficiary’s medical records:
- Beneficiary date of birth;
- Actual pack - year smoking history (number);
- Current smoking status, and for former smokers, the number of years since quitting smoking;
- Statement that the beneficiary is asymptomatic (no signs or symptoms of lung cancer); and
- National Provider Identifier (NPI) of the ordering practitioner.
Reading radiologist eligibility criteria:
- Board certification or board eligibility with the American Board of Radiology or equivalent organization;
- Documented training in diagnostic radiology and radiation safety;
- Involvement in the supervision and interpretation of at least 300 chest computed tomography acquisitions in the past 3 years;
- Documented participation in continuing medical education in accordance with current American College of Radiology standards; and
- Furnish lung cancer screening with LDCT in a radiology imaging facility that meets the radiology imaging facility eligibility criteria below.
Radiology imaging facility eligibility criteria:
- Performs LDCT with volumetric CT dose index (CTDIvol) of ≤ 3.0 mGy (milligray) for standard size patients (defined to be 5’ 7” and approximately 155 pounds) with appropriate reductions in CTDIvol for smaller patients and appropriate increases in CTDIvol for larger patients;
- Utilizes a standardized lung nodule identification, classification and reporting system;
- Makes available smoking cessation interventions for current smokers; and
- Collects and submits data to a CMS-approved registry for each LDCT lung cancer screening performed. The data collected and submitted to a CMS-approved registry must include, at minimum, all of the following elements:
| Data Type |
Minimum Required Data Elements |
|---|
Facility |
Identifier |
| Radiologist (reading) |
National Provider Identifier (NPI) |
| Patient |
Identifier |
| Ordering Practitioner |
National Provider Identifier (NPI) |
| CT scanner |
Manufacturer, Model. |
| Indication |
Lung cancer LDCT screening – absence of signs or symptoms of lung cancer |
| System |
Lung nodule identification, classification and reporting system |
| Smoking history |
Current status (current, former, never).
If former smoker, years since quitting.
Pack-years as reported by the ordering practitioner.
For current smokers, smoking cessation interventions available. |
| Effective radiation dose |
CT Dose Index (CTDIvol). |
| Screening |
Screen date Initial screen or subsequent screen |
All CMS-approved registries must have the capacity and capability to collect data from any Medicare-eligible imaging facility/department that furnishes lung cancer screening with LDCT, with a catchment area that includes all 50 States, United States Territories, and the District of Columbia. CMS will evaluate each entity interested in participating as a CMS-approved registry to determine if they are capable of meeting the registry and data collection requirements outlined in this national coverage determination, including:
- Establishment of a steering committee and a governance board for oversight of the registry;
- Registry management plan, including identification of key personnel;
- Operational plan and framework that describes mechanisms for collection and submission of data from imaging facilities to the registry;
- Registry catchment area;
- Mechanisms for the submission of registry data to CMS electronically;
- Mechanisms to collect information (e.g.; HICN) in order to permit linkage of registry data with external databases (e.g. Medicare claims data sets);
- Description of data management and data quality review methods, including validation;
- Use of CMS-approved standardized data dictionary;
- Mechanisms for submitting a list of facilities participating in the registry to CMS; and
- Quality assurance plan.
To apply to function as a CMS-approved registry, interested entities must submit a letter of interest along with detailed supporting information about how the interested entity is able to meet the requirements outlined in
this national coverage determination to the following address or via email to caginquiries@cms.hhs.gov.
Centers for Medicare & Medicaid Services
Center for Clinical Standards and Quality
Director, Coverage and Analysis Group
ATTN: Lung Cancer LDCT Screening
Mail Stop: S3-02-01
7500 Security Blvd.
Baltimore, MD 21244
Information regarding CMS-approved registries will be posted on the CMS website.
II. Background
Throughout this document we use numerous acronyms, some of which are not defined as they are presented in direct quotations. Please find below a list of these acronyms and corresponding full terminology.
AAFP – American Academy of Family Physicians
ACR – American College of Radiology
ALA – American Lung Association
AWV – annual wellness visit
CAT – computerized axial tomography
CCI – Charlson comorbidity index
CT – computed tomography
CTDIvol – computed tomography dose index
CXR – chest x-ray
DANTE – Detection and Screening of Early Lung Cancer by Novel Imaging Technology Molecular Assays
DLCST – Danish Lung Cancer Screening Trial
FDA – United States Food and Drug Administration
FDCA – Federal Food, Drug, and Cosmetic Act
JNCCN – Journal of the National Comprehensive Cancer Network
LC – lung cancer
LDCT – low dose computed tomography
MCBS – Medicare Current Beneficiary Survey
mGy – milligray
mSv – millisievert
MILD – Multicentric Italian Lung Detection
NCCN – National Comprehensive Cancer Network
NCI – National Cancer Institute
NCRP – National Council on Radiation Protection and Measurements
NIH – National Institutes of Health
NLST – National Lung Screening Trial
NSCLC – non-small cell lung cancer
PLCO – Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial
PMA – premarket approval application
PPV – positive predictive value
RCT – randomized control trial
SEER – Surveillance, Epidemiology, and End Results
USPSTF – United States Preventive Services Task Force
CMS initiated this national coverage determination (NCD) to consider coverage under the Medicare Program for lung cancer screening with low dose computed tomography (LDCT). The scope of our review is limited to the consideration of screening for lung cancer with LDCT. Diagnostic CTs are outside the scope of this national coverage analysis.
Lung cancer (LC) is the third most common cancer and the leading cause of cancer deaths in the United States. It is an important issue for the Medicare population due to the age at diagnosis and at death. In 2013, the National Cancer Institute (NCI) estimated that the number of new cases is over 220,000, with a median age at diagnosis of 70 years. Cancer of the lung and bronchus accounted for over 150,000 deaths in 2013 (more than the total number of deaths from colon, breast and prostate cancer combined) with a median age at death of 72 years.
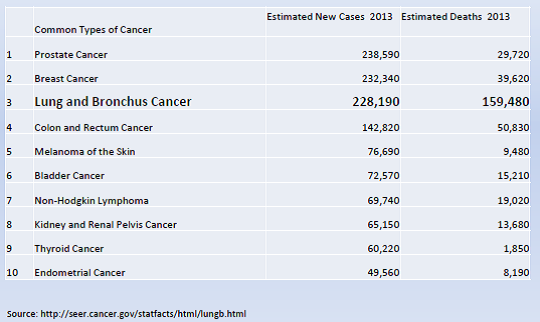
Overall mortality rates for lung and bronchus cancer have decreased only slightly over the past decade. The majority of cases are still diagnosed at a late stage with a low five-year relative survival. (NIH/NCI/SEER 2013).
The National Institutes of Health (NIH) National Cancer Institute (NCI) describes computed tomography (CT) as “an imaging procedure that uses special x-ray equipment to create detailed pictures, or scans, of areas inside the body. It is also called computerized tomography and computerized axial tomography (CAT). Most modern CT machines take continuous pictures in a helical (or spiral) fashion rather than taking a series of pictures of individual slices of the body, as the original CT machines did. Helical CT has several advantages over older CT techniques: it is faster, produces better 3-D pictures of areas inside the body, and may detect small abnormalities better. The newest CT scanners, called multislice CT or multidetector CT scanners, allow more slices to be imaged in a shorter period of time.” (NIH/NCI; http://www.cancer.gov/cancertopics/factsheet/Detection/CT)
The United States Food and Drug Administration (FDA) describes the mechanisms of a CT scanner as follows (also see image below): “A motorized table moves the patient through a circular opening in the CT imaging system. While the patient is inside the opening, an X-ray source and a detector assembly within the system rotate around the patient. A single rotation typically takes a second or less. During rotation, the X-ray source produces a narrow, fan-shaped beam of X-rays that passes through a section of the patient's body. Detectors in rows opposite the X-ray source register the X-rays that pass through the patient's body as a snapshot in the process of creating an image. Many different "snapshots" (at many angles through the patient) are collected during one complete rotation. For each rotation of the X-ray source and detector assembly, the image data are sent to a computer to reconstruct all of the individual "snapshots" into one or multiple cross-sectional images (slices) of the internal organs and tissues.”
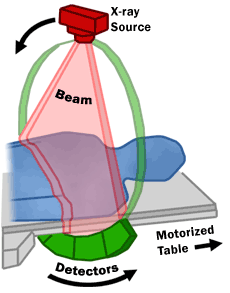
(US Food and Drug Administration (FDA); http://www.fda.gov/Radiation-EmittingProducts/RadiationEmittingProductsandProcedures/MedicalImaging/MedicalX-Rays/ucm115317.htm)
LDCT is a chest CT scan performed at acquisition settings to minimize radiation exposure compared to a standard chest CT (Smith-Bindman, 2009). Prasad et al. (2002) noted: “[s]everal factors influence patient radiation dose from CT including tube voltage, tube current, scanning time, pitch, slice thickness, and scanning volume. Radiation dose is linearly related to tube current, scanning time, and scan volume and inversely related to pitch. Although scanning times are decreased on modern helical CT scanners, the radiation dose associated with helical scanners is greater than that of other imaging procedures because of the increased tube current and volume of tissue irradiated.”
While LDCT reduces radiation exposure, image quality is also reduced which in turn may influence readability. Bankier and Tack (2010) noted: “[t]he concept of reducing the radiation dose in chest CT was first introduced by Naidich et al., (1990) who reduced the tube current on incremental 10-mm collimation CT and showed that with tube current settings as low as 20 mAs, the image quality is sufficient for assessing the lung parenchyma. Although these images were sufficient for diagnostically assessing the lung parenchyma, the increased image noise resulted in marked degradation of the quality of images on mediastinal window settings.”
Given the burden of lung cancer on the United States population, a suitable screening test for lung cancer has been sought for many years. The earliest studies were conducted in the 1960’s and 1970’s, testing sputum cytology and chest radiography or a combination of both. The initial studies using sputum cytology and/or chest radiography tests failed to conclusively demonstrate improvements in health outcomes, leading the United States Preventive Services Task Force (USPSTF) to give lung cancer screening a grade D recommendation in 1985 and again in 1996 (Humphrey, 2004). Low dose helical computed tomography began being considered for use as a screening modality starting in the 1990’s. Naidich et al. (1990) noted: “[a]lthough further evaluation is necessary, the potential of low-dose CT for use in the pediatric population in particular, as well as for screening in patients at high risk for developing lung cancer, is apparent.”
With accumulation of evidence on LDCT screening from studies in the late 1990s and early 2000s, the USPSTF updated their recommendation for lung cancer screening to a grade I (insufficient) recommendation and “found there was insufficient evidence to either recommend for or against routinely screening asymptomatic persons for lung cancer with either low-dose computed tomography (LDCT), chest x-ray (CXR), sputum cytology, or a combination of these tests (I statement)” (Humphrey et al., 2013). The USPSTF noted that “screening with LDCT, CXR, or sputum cytology can detect lung cancer at an earlier stage than lung cancer would be detected in an unscreened population; however, the USPSTF found poor evidence that any screening strategy for lung cancer decreases mortality” (USPSTF, 2004). As with many technologies, the initial studies on lung cancer screening with LDCT were observational case-control and cohort studies to generate hypotheses, show feasibility, and to identify the appropriate screening population.
In 2011, the results of the NCI-sponsored National Lung Screening Trial (NLST) were published. The NLST showed that people aged 55 to 74 years with a history of heavy smoking are 20 percent less likely to die from lung cancer if they are screened with low dose helical CT than with standard screening chest x-rays. Previous studies had shown that screening with standard chest x-rays does not reduce the mortality rate from lung cancer. The NLST identified risks as well as benefits. For example, people screened with low dose helical CT had a higher overall rate of false-positive results (that is, findings that appeared to be abnormal even though no cancer was present), leading to a higher rate of invasive procedures (such as bronchoscopy or biopsy), and serious complications from such invasive procedures, than those screened with standard x-rays. (NIH/NCI at http://www.cancer.gov/cancertopics/factsheet/Detection/CT)
In 2014, Moyer and colleagues, on behalf of the USPSTF, released updated recommendations for lung cancer screening: “The USPSTF recommends annual screening for lung cancer with low-dose computed tomography in adults aged 55 to 80 years who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery. (B recommendation)” (Moyer for the USPSTF, 2014).
III. History of Medicare Coverage
Pursuant to §1861(ddd) of the Social Security Act, the Secretary may add coverage of "additional preventive services" if certain statutory requirements are met. Our regulations provide:
42 CFR §410.64 Additional preventive services
(a) Medicare Part B pays for additional preventive services not described in paragraph (1) or (3) of the definition of “preventive services” under §410.2, that identify medical conditions or risk factors for individuals if the Secretary determines through the national coverage determination process (as defined in section 1869(f)(1)(B) of the Social Security Act) that these services are all of the following:
(1) Reasonable and necessary for the prevention or early detection of illness or disability.
(2) Recommended with a grade of A or B by the United States Preventive Services Task Force.
(3) Appropriate for individuals entitled to benefits under part A or enrolled under Part B.
(b) In making determinations under paragraph (a) of this section regarding the coverage of a new preventive service, the Secretary may conduct an assessment of the relation between predicted outcomes and the expenditures for such services and may take into account the results of such an assessment in making such national coverage determinations.
A. Current Request
CMS received two formal requests for a national coverage determination (NCD) for lung cancer screening with LDCT, one from Peter B. Bach (Director, Center for Health Policy and Outcomes, Memorial Sloan-Kettering Cancer Center), and another from Laurie Fenton Ambrose (President & CEO, Lung Cancer Alliance). The formal request letters can be viewed via the tracking sheet for this NCA on the CMS website at http://www.cms.gov/medicare-coverage-database/details/nca-tracking-sheet.aspx?NCAId=274.
B. Benefit Category
In order to be covered by Medicare, an item or service must fall within one or more benefit categories contained within Part A or Part B and must not be otherwise excluded from coverage. Since January 1, 2009, CMS is authorized to cover "additional preventive services" if certain statutory requirements are met as provided under §1861(ddd) of the Social Security Act.
IV. Timeline of Recent Activities
| Date |
Action |
| February 10, 2014 |
CMS initiates this national coverage analysis. A 30-day public comment period began. |
| March 12, 2014 |
The 30-day public comment period closed. |
| April 30, 2014 |
CMS convened a meeting of the Medicare Evidence Development & Coverage Advisory Committee concerning the use of LDCT screening for lung cancer. |
| November 10, 2014 |
CMS posted a proposed national coverage determination for screening for lung cancer with LDCT. A 30-day public comment period on the proposed decision began. |
| December 10, 2014 |
The 30-day public comment period on the proposed decision closed. |
[posting date] |
CMS posted the final national coverage determination. |
V. Food and Drug Administration (FDA) Status
Currently, CT imaging systems and post-processing software go through the 510(k) process at the FDA to obtain clearance for commercial distribution. To obtain 510(k) clearance, the sponsor must demonstrate that the device is substantially equivalent to legally marketed predicate devices marketed in interstate commerce prior to May 28, 1976, the enactment date of the Medical Device Amendments, or to devices that have been reclassified in accordance with the provisions of the Federal Food, Drug, and Cosmetic Act (FDCA) that do not require approval of a premarket approval application (PMA).
CT devices were on the market prior to the passage of the Medical Device Amendments. They were originally indicated for general cross sectional imaging of the body. This includes the lungs and other specific organs. Subsequent modifications based on either additional built-in processing or on post-processing have expanded the breadth of CT images and with that, their use.
Counseling services are not generally under the purview of the FDA.
VI. General Methodological Principles
When making national coverage determinations concerning additional preventive services, CMS applies the statutory criteria in §1861(ddd) of the Social Security Act and regulations at 42 CFR 410.64, and evaluates relevant clinical evidence to determine whether or not the service is reasonable and necessary for the prevention or early detection of illness or disability, is recommended with a grade of A or B by the USPSTF, and is appropriate for individuals entitled to benefits under Part A or enrolled under Part B of the Medicare program.
CMS uses the initial public comments to inform its proposed national coverage determination. CMS responds in detail to the public comments on a proposed national coverage determination when issuing the final national coverage determination. Public comments sometimes cite published clinical evidence and give CMS useful information. Public comments that give information on unpublished evidence such as the results of individual practitioners or patients are less rigorous and therefore less useful for making a coverage determination. Public comments that contain personal health information will not be made available to the public.
VII. Evidence
A. Introduction
While a detailed discussion of screening is beyond the scope of this discussion, the basic parameters were established many years ago and are still well accepted to date. In 1968, Wilson and Jungner reported criteria to consider for screening:
- The condition being screened for should be an important health problem,
- The natural history of the condition should be well understood,
- There should be a detectable early stage,
- Treatment at an early stage should be of more benefit than at a later stage,
- A suitable test should be devised for the early stage,
- The test should be acceptable,
- Intervals for repeating the test should be determined,
- Adequate health service provision should be made for the extra clinical workload resulting from screening,
- The risks, both physical and psychological, should be less than the benefits,
- The costs should be balanced against the benefits.
(Wilson JMG, Jungner G. Principles and Practice of Screening for Disease. World Health Organization, 1968)
Health outcomes, benefits, and risks are important considerations. As Cochrane and Holland (1971) further noted, evidence on health outcomes, i.e., "evidence that screening can alter the natural history of disease in a significant proportion of those screened," is important in the consideration of screening tests since individuals are asymptomatic and "the practitioner initiates screening procedures."
The evaluation of screening tests has been largely standardized in the medical and scientific communities, and the "value of a screening test may be assessed according to the following criteria:
- “Simplicity. In many screening programmes more than one test is used to detect one disease, and in a multiphasic programme the individual will be subjected to a number of tests within a short space of time. It is therefore essential that the tests used should be easy to administer and should be capable of use by para-medical and other personnel.
- Acceptability. As screening is in most instances voluntary and a high rate of co-operation is necessary in an efficient screening programme, it is important that tests should be acceptable to the subjects.
- Accuracy. The test should give a true measurement of the attribute under investigation.
- Cost. The expense of screening should be considered in relation to the benefits resulting from the early detection of disease, i.e., the severity of the disease, the advantages of treatment at an early stage and the probability of cure.
- Precision (sometimes called repeatability). The test should give consistent results in repeated trials.
- Sensitivity. This may be defined as the ability of the test to give a positive finding when the individual screened has the disease or abnormality under investigation.
- Specificity. This may be defined as the ability of the test to give a negative finding when the individual screened does not have the disease or abnormality under investigation”.
(Cochrane A and Holland W. Validation of screening procedures. British Medical Bulletin 1971;27(1):3-8. PMID: 5100948).
B. United States Preventive Services Task Force (USPSTF)
The USPSTF recommendation for lung cancer screening with LDCT (December 2013) states:
- The USPSTF recommends annual screening for lung cancer with low-dose computed tomography in adults ages 55 to 80 years who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery. Grade: B recommendation.
The USPSTF assigns one of five letter grades to each of its recommendations (A, B, C, D, I). The following tables from the USPSTF website provide the current grade definitions and descriptions of levels of certainty.
(http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm)
Grade Definitions After July 2012
| Grade |
Definition |
Suggestions for Practice |
| A |
The USPSTF recommends the service. There is high certainty that the net benefit is substantial. |
Offer or provide this service. |
| B |
The USPSTF recommends the service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. |
Offer or provide this service. |
| C |
The USPSTF recommends selectively offering or providing this service to individual patients based on professional judgment and patient preferences. There is at least moderate certainty that the net benefit is small. |
Offer or provide this service for selected patients depending on individual circumstances. |
| D |
The USPSTF recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. |
Discourage the use of this service. |
| I Statement |
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
Read the clinical considerations section of USPSTF Recommendation Statement. If the service is offered, patients should understand the uncertainty about the balance of benefits and harms. |
| Level of Certainty |
Description |
|---|
| High |
The available evidence usually includes consistent results from well-designed, well-conducted studies in representative primary care populations. These studies assess the effects of the preventive service on health outcomes. This conclusion is therefore unlikely to be strongly affected by the results of future studies. |
| Moderate |
The available evidence is sufficient to determine the effects of the preventive service on health outcomes, but confidence in the estimate is constrained by such factors as:
The number, size, or quality of individual studies.
Inconsistency of findings across individual studies.
Limited generalizability of findings to routine primary care practice.
Lack of coherence in the chain of evidence.
As more information becomes available, the magnitude or direction of the observed effect could change, and this change may be large enough to alter the conclusion. |
| Low |
The available evidence is insufficient to assess effects on health outcomes. Evidence is insufficient because of: The limited number or size of studies. Important flaws in study design or methods. Inconsistency of findings across individual studies. Gaps in the chain of evidence. Findings not generalizable to routine primary care practice. Lack of information on important health outcomes. More information may allow estimation of effects on health outcomes. |
* The USPSTF defines certainty as "likelihood that the USPSTF assessment of the net benefit of a preventive service is correct." The net benefit is defined as benefit minus harm of the preventive service as implemented in a general, primary care population. The USPSTF assigns a certainty level based on the nature of the overall evidence available to assess the net benefit of a preventive service.
C. Literature Search
CMS searched PubMed from May 2004 to August 2014. General keywords included screening low dose CT and lung cancer. Publications that presented original data on screening were considered. Abstracts, animal studies and non-English language publications were excluded.
D. Discussion of Evidence
Question 1: Is the evidence sufficient to determine that screening for lung cancer with low dose computed tomography is recommended with a grade of A or B by the United States Preventive Services Task Force?
Question 2: Is the evidence sufficient to determine that screening for lung cancer with low dose computed tomography is reasonable and necessary for the prevention or early detection of illness or disability?
Question 3: Is the evidence sufficient to determine that screening for lung cancer with low dose computed tomography is appropriate for Medicare beneficiaries?
1. External technology assessments
Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, Byers T, Colditz GA, Gould MK, Jett JR, Sabichi AL, Smith-Bindman R, Wood DE, Qaseem A, Detterbeck FC. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012 Jun 13;307(22):2418-29. doi: 10.1001/jama.2012.5521. PMID: 22610500
Bach and colleagues reported the results of a systematic review of the evidence regarding the benefits and harms of lung cancer screening using LDCT. Studies were eligible “if they involved either an RCT using LDCT screening for lung cancer in one intervention group or a noncomparative cohort study of LDCT screening, provided they reported at least 1 of the following outcomes: lung cancer−specific or all-cause mortality, nodule detection rate, frequency of additional imaging, frequency of invasive diagnostic procedures (e.g., needle or bronchoscopic biopsy, surgical biopsy, surgical resection), complications from the evaluation of suspected lung cancer, or the rate of smoking cessation or reinitiation.” Searching from 1996 to April 2012, eight randomized trials and 13 cohort studies met criteria. Three trials provided evidence on mortality. The NLST (NLST Team, 2012; n = 53,454) was a randomized trial that compared LDCT to chest radiography.
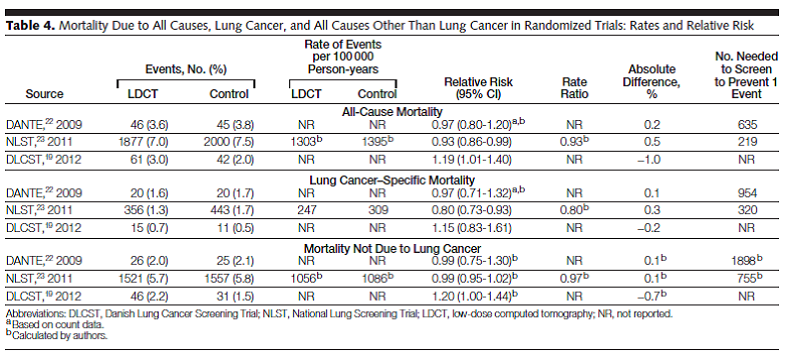
Table 4. Page 2422. JAMA. 2012 Jun 13;307(22):2418-29.
The authors concluded: “Screening a population of individuals at a substantially elevated risk of lung cancer most likely could be performed in a manner such that the benefits that accrue to a few individuals outweigh the harms that many will experience. However, there are substantial uncertainties regarding how to translate that conclusion into clinical practice.”
Humphrey LL, Teutsch S, Johnson M; U.S. Preventive Services Task Force. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2004 May 4;140(9):740-53. PMID: 15126259
Humphrey and colleagues reported the results of a systematic evidence review on lung cancer screening that provided the evidence base for the 2004 USPSTF recommendation. Studies on lung cancer screening using sputum cytology, chest radiology and computed tomography were reviewed. For LDCT, several observational studies were reviewed (presented in Table 4). There were no randomized controlled trials.
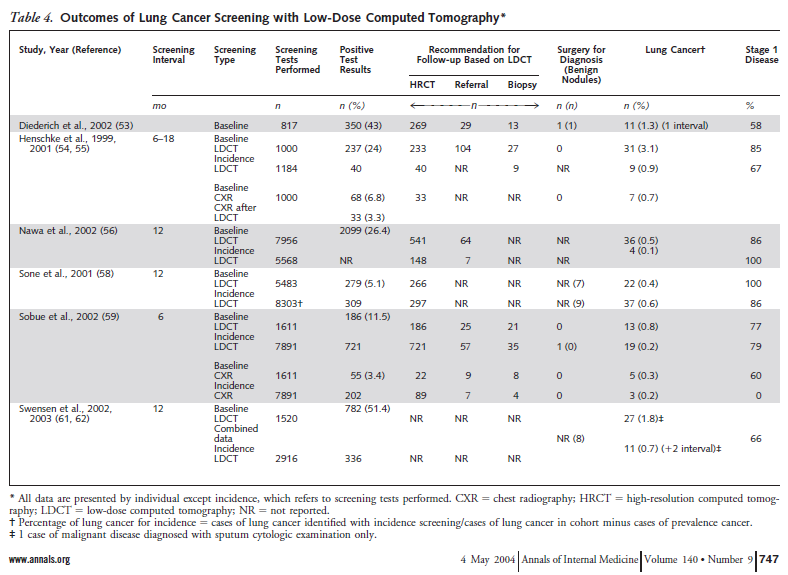
Table 4. Humphrey et al. Ann Intern Med. 2004 May 4;140(9):747. www.annals.org
Humphrey and colleagues concluded: “Current data do not support screening for lung cancer with any method. These data, however, are also insufficient to conclude that screening does not work, particularly in women. Two randomized trials of screening with chest radiography or low-dose CT are currently under way and will better inform lung cancer screening decisions.”
Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, Zakher B, Fu R, Slatore CG. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2013 Sep 17;159(6):411-20. doi: 10.7326/0003-4819-159-6-201309170-00690. PMID: 23897166
Humphrey and colleagues reported the results of a systematic review to re-evaluate the effectiveness of LDCT screening for lung cancer for the USPSTF. Trials and cohort studies that evaluated screening or treatment interventions for lung cancer that reported health outcomes were eligible. Searching from 2000 to May 2013, seven trials met criteria, with four trials that were included in the review because they reported results for both intervention and control groups. The NLST (NLST team, 2012; n = 53,454) compared LDCT to chest radiography. The authors stated: “One large good-quality trial reported that screening was associated with significant reductions in lung cancer (20 %) and all-cause (6.7 %) mortality. Three small European trials showed no benefit of screening. Harms included radiation exposure, overdiagnosis, and a high rate of false positive findings that typically were resolved with further imaging. Smoking cessation was not affected. Incidental findings were common.”
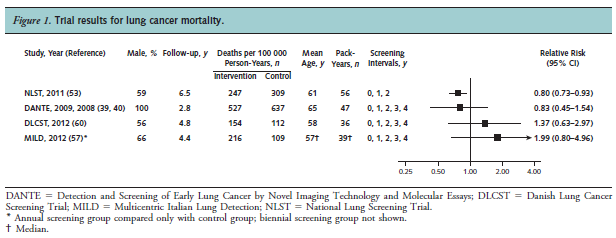
Figure 1. Page 414. Ann Intern Med. 2013 Sep 17;159(6):411-20.
The authors concluded: “Strong evidence shows that LDCT screening can reduce lung cancer and all-cause mortality. The harms associated with screening must be balanced with the benefits.”
Manser R, Lethaby A, Irving LB, Stone C, Byrnes G, Abramson MJ, Campbell D. Screening for lung cancer. Cochrane Database Syst Rev. 2013 Jun 21;6:CD001991. doi: 10.1002/14651858.CD001991.pub3. PMID: 23794187
Manser and colleagues reported the results of a Cochrane systematic review “to determine whether screening for lung cancer, using regular sputum examinations, chest radiography or CT scanning of the chest, reduces lung cancer mortality.” Controlled trials were included. This report updated the original review performed in 1999.
The authors reported: “We included nine trials in the review (eight randomised controlled studies and one controlled trial) with a total of 453,965 subjects. In one large study that included both smokers and non-smokers comparing annual chest x-ray screening with usual care there was no reduction in lung cancer mortality (RR 0.99, 95 % CI 0.91 to 1.07). In a meta-analysis of studies comparing different frequencies of chest x-ray screening, frequent screening with chest x-rays was associated with an 11 % relative increase in mortality from lung cancer compared with less frequent screening (RR 1.11, 95 % CI 1.00 to 1.23); however several of the trials included in this meta-analysis had potential methodological weaknesses. We observed a non-statistically significant trend to reduced mortality from lung cancer when screening with chest x-ray and sputum cytology was compared with chest x-ray alone (RR 0.88, 95% CI 0.74 to 1.03). There was one large methodologically rigorous trial in high-risk smokers and ex-smokers (those aged 55 to 74 years with &#≥ 30 pack-years of smoking and who quit ≤ 15 years prior to entry if ex-smokers) comparing annual low-dose CT screening with annual chest x-ray screening; in this study the relative risk of death from lung cancer was significantly reduced in the low-dose CT group (RR 0.80, 95% CI 0.70 to 0.92).”

Page 19. Cochrane Database Syst Rev. 2013 Jun 21;6:CD001991.
The authors concluded: “The current evidence does not support screening for lung cancer with chest radiography or sputum cytology. Annual low-dose CT screening is associated with a reduction in lung cancer mortality in high-risk smokers but further data are required on the cost effectiveness of screening and the relative harms and benefits of screening across a range of different risk groups and settings.”
Prosch H, Schaefer-Prokop C. Screening for lung cancer. Curr Opin Oncol. 2014 Mar;26(2):131-7. doi: 10.1097/CCO.0000000000000055. PMID: 24441507
Prosch and Schaefer-Prokop performed a systematic review of recent randomized controlled trials of lung cancer screening using LDCT. Included trials were the NLST, the Detection And screening of early lung cancer by Novel imaging TEchnology and molecular assays (DANTE) trial, the Multicentric Italian Lung Detection (MILD) trial, and the Danish Lung Cancer Screening Trial (DLCST). They reported: “The National Lung Screening Trial (NLST) was the first study that provided statistical evidence that LD-CT screening for lung cancer significantly reduces lung cancer mortality by 20 %. Three statistically underpowered European trials could not confirm the positive effect of LD-CT screening on lung cancer mortality. Major obstacles in lung cancer screening are overdiagnosis and the large number of false positive results. In the NLST, more than 24 % of the screens were positive, most of which (96.4 %) proved to be benign in nature. Optimized protocols for the workup of detected nodules may help to reduce the number of false-positive screens.” They further noted: “Long-term follow-up data are still anticipated on the European screening trials. Furthermore, data on the extent of the potential dangers of LD-CT screening, such as overdiagnosis, false-positive results, and the effect of cumulative radiation dose, have yet to be investigated thoroughly.”

Table 1. Page 133. Curr Opin Oncol. 2014 Mar;26(2):131-7.
2. Internal technology assessment
Infante M, Cavuto S, Lutman FR, Brambilla G, Chiesa G, Ceresoli G, Passera E, Angeli E, Chiarenza M, Aranzulla G, Cariboni U, Errico V, Inzirillo F, Bottoni E, Voulaz E, Alloisio M, Destro A, Roncalli M, Santoro A, Ravasi G; DANTE Study Group. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009 Sep 1;180(5):445-53. doi: 10.1164/rccm.200901-0076OC. Epub 2009 Jun 11. PMID: 19520905
Infante and colleagues reported results of the DANTE randomized trial designed “to explore the effect of screening with low-dose spiral computed tomography (LDCT) on lung cancer mortality.” Men aged 60 to 74 years with &#≥ 20 pack-years smoking history were eligible. Patients with comorbidities that limited life expectancy to < 5 years, had a previous history of malignancy, or who were unable to comply with study protocols were excluded. Primary outcome was lung cancer mortality. Secondary outcomes included all-cause mortality, lung cancer incidence, stage and resectability. Between March 2001 and February 2006, 2811 participants were randomly assigned and 2472 were enrolled to yearly LDCT screening for four years (n = 1276) or yearly medical care control (n = 1196) at three sites in Italy. Mean age was 64 years. About 56 % were active smokers at baseline. Median follow-up was 33.7 months. Mean pack-years smoking was 47.
The authors reported 20 (1.6 %) lung cancer deaths in the LDCT group and 20 (1.7 %) in the control group (p = 0.84) and 46 (3.6 %) total deaths in the LDCT group and 45 (3.8 %) in the control group (p = 0.83). They concluded: “possible overdiagnosis, false positives, hazards of downstream investigation procedures, and cost issues make the results of randomized studies critically important in establishing a proper public health policy, and the final results from all ongoing randomized trials are awaited. In the meantime, continued application of current policies is supported by our data, and screening for LC with LDCT should not be advertised or proposed to high-risk subjects outside research programs.”
National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011 Aug 4;365(5):395-409. doi: 10.1056/NEJMoa1102873. Epub 2011 Jun 29. PMID: 21714641
The NCI-sponsored NLST was a randomized trial “to determine whether screening with low-dose CT, as compared with chest radiography, would reduce mortality from lung cancer among high-risk persons.” From August 2002 to April 2004, 53,454 individuals who were “between 55 and 74 years of age at the time of randomization, had a history of cigarette smoking of at least 30 pack-years, and, if former smokers, had quit within the previous 15 years” were enrolled at 33 U.S. centers and randomized to screening with 3 annual LDCT (n = 26,722) or three annual chest radiography (n = 26,732). Individuals who “had previously received a diagnosis of lung cancer, had undergone chest CT within 18 months before enrollment, had hemoptysis, or had an unexplained weight loss of more than 6.8 kg (15 lb) in the preceding year were excluded.” All screening tests were performed and interpreted according to protocol (NLST study design, 2011). It was “estimated that each NLST low-dose CT resulted in an average effective dose of 1.5 mSv, whereas the effective dose from conventional chest CT varies considerably in clinical practice but is on the order of 8 mSv” (NLST study design, 2011). The primary outcome was lung cancer mortality. Secondary outcomes included all-cause mortality and lung cancer incidence. Median age was approximately 61 years with 26.6 % of participants being 65 years and older. Men comprised 59 % of the study. Ninety one percent were white. Forty eight percent were current smokers. Median follow-up was 6.5 years. Adherence to all three screenings was 95 % in the LDCT group and 93 % in the radiography group.
The authors reported “[t]here were 247 deaths from lung cancer per 100,000 person-years in the low-dose CT group and 309 deaths per 100,000 person-years in the radiography group, representing a relative reduction in mortality from lung cancer with low-dose CT screening of 20.0 % (95 % CI, 6.8 to 26.7; P = 0.004). The rate of death from any cause was reduced in the low-dose CT group, as compared with the radiography group, by 6.7 % (95 % CI, 1.2 to 13.6; P = 0.02).”

Table 7. page 407. N Engl J Med. 2011 Aug 4;365(5):395-409.
The authors concluded “[s]creening with the use of low-dose CT reduces mortality from lung cancer.”
Pastorino U, Rossi M, Rosato V, Marchianò A, Sverzellati N, Morosi C, Fabbri A, Galeone C, Negri E, Sozzi G, Pelosi G, La Vecchia C. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev. 2012 May;21(3):308-15. doi: 10.1097/CEJ.0b013e328351e1b6. PMID: 22465911
Pastorino and colleagues reported the results of the MILD randomized trial “to evaluate the impact on mortality of early lung cancer detection through LDCT at annual or biennial intervals versus no screening.” Individuals who were &#≥ 49 years of age with &#≥ 20 pack-year smoking history and no history of cancer within the past five years were included. No other exclusion criteria were reported. From September 2005 to January 2011, 4099 participants were enrolled and randomly assigned to annual LDCT screening (n = 1190), biennial LDCT screening (n = 1186) and no screening control group with smoking cessation interventions and pulmonary function tests (n = 1723) at one site in Italy (trial was initially designed to be conducted at multiple centers but limited to one site due to funding). Primary outcome was lung cancer mortality. Secondary outcomes included all-cause mortality and lung cancer incidence. Median age was 57 years. Men comprised 67 % of the study population. At baseline, there were more current smokers in the control group (89.7 %) compared to the LDCT screening groups (68.3 % annual and 68.9 % biennial). Median pack-years smoking was 39.
The authors reported: “The cumulative 5-year lung cancer incidence rate was 311/100 000 in the control group, 457 in the biennial, and 620 in the annual LDCT group (P = 0.036); lung cancer mortality rates were 109, 109, and 216/100 000 (P = 0.21), and total mortality rates were 310, 363, and 558/100 000, respectively (P = 0.13).” They concluded: “There was no evidence of a protective effect of annual or biennial LDCT screening. Furthermore, a meta-analysis of the four published randomized trials [DANTE (Infante), DLCST (Saghir), MILD, NLST] showed similar overall mortality in the LDCT arms compared with the control arm.” “The effect on lung cancer mortality remains significant (relative risk 0.82, 95 % CI 0.73 – 0.93) but the value of disease-specific mortality as the only endpoint appears questionable for two reasons: the assessment of the real cause of death can be very difficult in heavy smokers because of complex comorbidity; a shift in the cause of death from one disease to another is frequent in screened populations and hence potentially misleading.”
Saghir Z1, Dirksen A, Ashraf H, Bach KS, Brodersen J, Clementsen PF, Døssing M, Hansen H, Kofoed KF, Larsen KR, Mortensen J, Rasmussen JF, Seersholm N, Skov BG, Thorsen H, Tønnesen P, Pedersen JH. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax. 2012 Apr;67(4):296-301. doi: 10.1136/thoraxjnl-2011-200736. Epub 2012 Jan 27.PMID: 22286927.
Saghir and colleagues reported the results of a randomized controlled trial “to evaluate if annual low dose CT screening [for 5 annual screenings] can reduce lung cancer mortality by more than 25 %” (Pederson, 2009). Inclusion criteria were age 50-70 years, current or former smokers with at least 20 pack-years of smoking history, ability to climb two flights of stairs (36 steps) without pausing. Exclusion criteria were “weight over 130 kg, history of cancer diagnosis and treatment, lung tuberculosis, illness that would shorten life expectancy to < 10 years and chest CT received during the last year for any reason.” Primary outcome was lung cancer mortality. Secondary outcomes included overall mortality, number of lung cancers, stage and health economic evaluations. From October 2004 to March 2006, 4104 participants were enrolled and randomly assigned to CT screening group (five annual LDCT; n = 2052) or control group (no screening) (n = 2052) at one site in Denmark. “All participants had an annual visit at the screening clinic, where lung function tests were performed, and questionnaires concerning health, lifestyle, smoking habits and psychosocial consequences of screening were completed.” Median age was not reported (peak number of patients were between 55-59 years). Men comprised 55 % of the study population. Median follow-up was 4.81 years.
The authors reported: “At the end of screening, 61 patients died in the screening group and 42 in the control group (p = 0.059). 15 and 11 died of lung cancer, respectively (p = 0.428).” They concluded: “CT screening for lung cancer brings forward early disease, and at this point no stage shift or reduction in mortality was observed. More lung cancers were diagnosed in the screening group, indicating some degree of over diagnosis and need for longer follow-up.”
3. Medicare Evidence Development & Coverage Advisory Committee (MEDCAC)
On April 30, 2014, a Medicare Evidence Development & Coverage Advisory Committee (MEDCAC) meeting was convened to discuss the body of evidence, hear presentations, consider public comments, and make recommendations to CMS regarding the currently available evidence related to early detection (screening) of lung cancer with LDCT in asymptomatic adults with histories of significant smoking. A CMS representative presented a basic background of lung cancer screening; how Medicare's consideration of preventive services is statutorily different than CMS’ consideration of evaluation and management services; and read the voting and discussion questions that would be considered by the panel. The panel heard presentations from four invited guest speakers, 16 scheduled speakers, and four members of the public.
The eight voting members on the panel voted on three questions (listed below) using a scale of one to five, with one representing a low confidence vote and five representing a high confidence vote. An average voting score of 2.5 represented intermediate confidence. The scores of the voting panel members were recorded and the average was calculated.
- How confident are you that there is adequate evidence to determine if the benefits outweigh the harms of lung cancer screening with LDCT [CT acquisition variables set to reduce exposure to an average effective dose of 1.5 mSv…] in the Medicare population?
- How confident are you that the harms of lung cancer screening with LDCT (average effective dose of 1.5 mSv) if implemented in the Medicare population will be minimized?
-
How confident are you that clinically significant evidence gaps remain regarding the use of LDCT (average effective dose of 1.5 mSv) for lung cancer screening in the Medicare population outside a clinical trial?
- Average score: 4.67. Since the resulting average voting score on this question showed at least intermediate confidence (score &#≥ 2.5), the panel was asked to discuss any significant gaps identified and how CMS might support their closure.
Information about the meeting, including the agenda, presentations from speakers, transcripts, and results of the voting questions are available on the CMS Website at: http://www.cms.gov/medicare-coverage-database/details/medcac-meeting-details.aspx?MEDCACId=68.
4. Evidence-based guidelines
Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013 May;143(5 Suppl):e78S-92S. doi: 10.1378/chest.12-2350. PMID: 23649455
Detterbeck and colleagues reported the American College of Chest Physicians guidelines based in part on the systematic review by Bach (2012). Specifically for lung cancer screening with LDCT:
“3.4.1. For smokers and former smokers who are age 55 to 74 and who have smoked for 30 pack-years or more and either continue to smoke or have quit within the past 15 years, we suggest that annual screening with LDCT should be offered over both annual screening with CXR or no screening, but only in settings that can deliver the comprehensive care provided to NLST participants (Grade 2B).” The guidelines also included the following remarks.
- “Counseling should include a complete description of potential benefits and harms, so the individual can decide whether to undergo LDCT screening.”
- “Screening should be conducted in a center similar to those where the NLST was conducted, with multidisciplinary coordinated care and a comprehensive process for screening, image interpretation, management of findings, and evaluation and treatment of potential cancers.”
- “A number of important questions about screening could be addressed if individuals who are screened for lung cancer are entered into a registry that captures data on follow-up testing, radiation exposure, patient experience, and smoking behavior.”
- “Quality metrics should be developed such as those in use for mammography screening, which could help enhance the benefits and minimize the harm for individuals who undergo screening.”
- “Screening for lung cancer is not a substitute for stopping smoking. The most important thing patients can do to prevent lung cancer is not smoke.”
- “The most effective duration or frequency of screening is not known.”
U.S. Preventive Services Task Force. Lung cancer screening: recommendation statement. Ann Intern Med. 2004 May 4;140(9):738-9. PMID: 15126258
In 2004, the USPSTF concluded that “the evidence is insufficient to recommend for or against screening asymptomatic persons for lung cancer with either low-dose computerized tomography (LDCT), chest x-ray (CXR), sputum cytology, or a combination of these tests. This is a grade I recommendation.” As reported in the analysis, “The USPSTF found fair evidence that screening with LDCT, CXR or sputum cytology can detect lung cancer at an earlier stage than lung cancer would be detected in an unscreened population; however, the USPSTF found poor evidence that any screening strategy for lung cancer decreases mortality. Because of the invasive nature of diagnostic testing and the possibility of a high number of false-positive tests in certain populations, there is potential for significant harms from screening. Therefore, the USPSTF could not determine the balance between the benefits and harms of screening for lung cancer.”
Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014 Mar 4;160(5):330-8. doi: 10.7326/M13-2771. PMID: 24378917
Moyer, on behalf of the USPSTF, reported a revised recommendation on lung cancer screening based on the review by Humphrey, et al. (2013) (see external technology assessments section above), concluding that “[t]he USPSTF recommends annual screening for lung cancer with low-dose computed tomography (LDCT) in adults aged 55 to 80 years who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery. (B recommendation)”
Wood DE, Eapen GA, Ettinger DS, Hou L, Jackman D, Kazerooni E, Klippenstein D, Lackner RP, Leard L, Leung AN, Massion PP, Meyers BF, Munden RF, Otterson GA, Peairs K, Pipavath S, Pratt-Pozo C, Reddy C, Reid ME, Rotter AJ, Schabath MB, Sequist LV, Tong BC, Travis WD, Unger M, Yang SC. Lung cancer screening. J Natl Compr Canc Netw. 2012 Feb;10(2):240-65. PMID: 22308518
Wood and colleagues reported the National Comprehensive Cancer Network® (NCCN®) guideline and consensus statement. The lung cancer panel recommended “helical LDCT screening for select patients at high risk for lung cancer based on the NLST results, nonrandomized studies, and observational data.” (2012)

Page 242. © JNCCN–Journal of the National Comprehensive Cancer Network | Volume 10 Number 2 | February 2012.
5. Professional Society Recommendations
American Academy of Family Physicians. Lung cancer clinical recommendations. Accessed May 12, 2014. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/lung-cancer.html.
The AAFP concluded that “the evidence is insufficient to recommend for or against screening for lung cancer with low-dose computed tomography (LDCT) in persons at high risk for lung cancer based on age and smoking history. (2013) (Grade: I recommendation)”
American Lung Association. Providing guidance on lung cancer screening to patients and physicians. April 23, 2012. Accessed May 12, 2014. Available at: http://www.lung.org/lung-disease/lung-cancer/lung-cancer-screening-guidelines/lung-cancer-screening.pdf
The American Lung Association “convened a Lung Cancer Screening Committee, chaired by Jonathan Samet, MD, MS, to review the current scientific evidence on cancer screening in order to assist the ALA in offering the best possible guidance to the public and those suffering from lung disease.” “The Committee acknowledges that cancer screening is associated with both benefits and risks and unfortunately, the NLST could not answer a number of questions on the advantages and safety of screening in the general population. In spite of this, the Committee provides the following interim recommendations:
- The best way to prevent lung cancer caused by tobacco use is to never start or quit smoking.
- Low-dose CT screening should be recommended for those people who meet NLST criteria:
- current or former smokers, aged 55 to 74 years
- a smoking history of at least 30 pack-years
- no history of lung cancer
- Individuals should not receive a chest X-ray for lung cancer screening
- Low-dose CT screening should NOT be recommended for everyone
- ALA should develop public health materials describing the lung cancer screening process in order to assist patients in talking with their doctors. This educational portfolio should include information that explains and clarifies for the public:
- the difference between a screening process and a diagnostic test
- the benefits, risks and costs (emotional, physical and economic)
- that not all lung cancers will be detected through use of low dose CT scanning
- A call to action should be issued to hospitals and screening centers to:
- establish ethical policies for advertising and promoting lung cancer CT screening services
- develop educational materials to assist patients in having careful and thoughtful discussions between patients and their physicians regarding lung cancer screening
- provide lung cancer screening services with access to multidisciplinary that can deliver the needed follow-up for evaluation of nodules.”
Jaklitsch MT, Jacobson FL, Austin JH, Field JK, Jett JR, Keshavjee S, MacMahon H, Mulshine JL, Munden RF, Salgia R, Strauss GM, Swanson SJ, Travis WD, Sugarbaker DJ. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012 Jul;144(1):33-8. doi: 10.1016/j.jtcvs.2012.05.060. PMID: 22710039
Jaklitsch and colleagues reported American Association for Thoracic Surgery guidelines for lung cancer screening using LDCT. “The American Association for Thoracic Surgery guidelines call for annual lung cancer screening with low-dose computed tomography screening for North Americans from age 55 to 79 years with a 30 pack-year history of smoking. Long-term lung cancer survivors should have annual low-dose computed tomography to detect second primary lung cancer until the age of 79 years. Annual low-dose computed tomography lung cancer screening should be offered starting at age 50 years with a 20 pack-year history if there is an additional cumulative risk of developing lung cancer of 5 % or greater over the following 5 years. Lung cancer screening requires participation by a subspecialty-qualified team. The American Association for Thoracic Surgery will continue engagement with other specialty societies to refine future screening guidelines.”
Kazerooni EA, Armstrong MR, Amorosa JK, Hernandez D, Liebscher LA, Nath H, McNitt-Gray MF, Stern EJ, Wilcox PA. ACR CT Accreditation Program and the Lung Cancer Screening Program Designation. J Am Coll Radiol. 2014 Nov 20. pii: S1546-1440(14)00633-4. doi: 10.1016/j.jacr.2014.10.002. [Epub ahead of print] PMID: 25455196
The authors reported the current position statement of the American College of Radiology: “The ACR strongly supports the use of LDCT for lung cancer screening and believes that the ability of this technique to reduce mortality depends on appropriate patient selection, the performance of high-quality, low–radiation exposure LDCT examinations interpreted by qualified physicians, and a structured reporting and management system as the foundation for quality reporting and outcomes monitoring. Building on extensive ACR experience with breast cancer screening, including the comprehensive widespread use of BI-RADS®, imaging accreditation, quality metrics, and both clinical and research registries, the ACR has focused on expeditiously bringing tools to practicing radiologists to promote the dissemination of high-quality lung cancer screening.
The ACR-Society of Thoracic Radiology (STR) Practice Parameter for the Performance and Reporting of Lung Cancer Screening Thoracic CT 2014 (Resolution 4) provides guidance on indications and contraindications, specifications of the examination, interpretation and reporting, documentation and communication, and equipment specifications. The first edition of the ACR’s Lung-RADS presents a structured management, reporting, and audit schema for lung cancer screening practice and quality assurance (QA). In addition, the ACR has collected lung cancer screening resources on a single web page for individuals, practices, and institutions. Provided links include the 2014 CT protocols from The American Association of Physicists in Medicine, and smoking cessation resources, an important component of any lung cancer screening program. Lastly, as part of the ACR suite of registries under the National Radiology Data Registry, the ACR will launch the Lung Cancer Screening Practice Registry in early 2015. Use of the registry will enable providers to perform clinical practice audits and compare their cancer detection rates, false positives, and positive predictive value rates against benchmarks similar to the standards used in mammography. Participation will be very useful for assuring quality in individual radiology practices.”
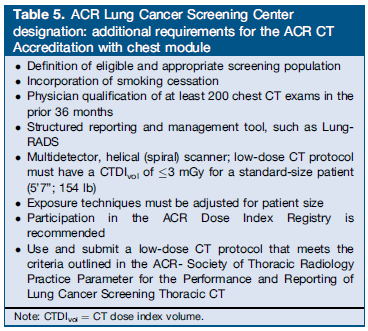
Table 5. Kazerooni et al. J Am Coll Radiol. 2014 Nov 20. pii: S1546-1440(14)00633-4. doi: 10.1016/j.jacr.2014.10.002. [Epub ahead of print] PMID: 25455196
Mazzone P, Powell CA, Arenberg D, Bach P, Detterbeck F, Gould M, Jaklitsch MT, Jett J, Naidich D, Vachani A, Wiener RS, Silvestri G. Components Necessary for High Quality Lung Cancer Screening: American College of Chest Physicians and American Thoracic Society Policy Statement. Chest. 2014 Oct 30. doi: 10.1378/chest.14-2500. [Epub ahead of print] PMID: 25356819
The authors presented a joint position statement endorsed by the American College of Chest Physicians, American Thoracic Society, American Association of Thoracic Surgery, American Cancer Society, and American Society of Preventive Oncology: “Lung cancer screening with a low dose chest CT scan can result in more benefit than harm when performed in settings committed to developing and maintaining high quality programs. This project aimed to identify the components of screening that should be a part of all lung cancer screening programs. To do so, committees with expertise in lung cancer screening were assembled by the Thoracic Oncology Network of the ACCP and the Thoracic Oncology Assembly of the ATS. Lung cancer program components were derived from evidence-based reviews of lung cancer screening, and supplemented by expert opinion. This statement was developed and modified based on iterative feedback of the committees. Nine essential components of a lung cancer screening program were identified. Within these components twenty one Policy Statements were developed and translated into criteria that could be used to assess the qualification of a program as a screening facility. Two additional Policy Statements related to the need for multi-society governance of lung cancer screening were developed. High quality lung cancer screening programs can be developed within the presented framework of nine essential program components outlined by our committees.” The components are:
Component 1: Who is offered lung cancer screening;
Component 2: How often, and for how long, to screen;
Component 3: How the CT is performed;
Component 4: Lung nodule identification;
Component 5: Structured reporting;
Component 6: Lung nodule management algorithms;
Component 7: Smoking cessation;
Component 8: Patient and provider education;
Component 9: Data collection;
Wender R, Fontham ET, Barrera E Jr, Colditz GA, Church TR, Ettinger DS, Etzioni R, Flowers CR, Gazelle GS, Kelsey DK, LaMonte SJ, Michaelson JS, Oeffinger KC, Shih YC, Sullivan DC, Travis W, Walter L, Wolf AM, Brawley OW, Smith RA. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013 Mar-Apr;63(2):107-17. doi: 10.3322/caac.21172. Epub 2013 Jan 11. PMID: 23315954
In 2013, Wender and colleagues, on behalf of the American Cancer Society, released lung cancer screening guidelines: “Findings from the National Cancer Institute’s National Lung Screening Trial established that lung cancer mortality in specific high-risk groups can be reduced by annual screening with low-dose computed tomography. These findings indicate that the adoption of lung cancer screening could save many lives. Based on the results of the National Lung Screening Trial, the American Cancer Society is issuing an initial guideline for lung cancer screening. This guideline recommends that clinicians with access to high-volume, high-quality lung cancer screening and treatment centers should initiate a discussion about screening with apparently healthy patients aged 55 years to 74 years who have at least a 30-pack-year smoking history and who currently smoke or have quit within the past 15 years. A process of informed and shared decision making with a clinician related to the potential benefits, limitations, and harms associated with screening for lung cancer with low-dose computed tomography should occur before any decision is made to initiate lung cancer screening. Smoking cessation counseling remains a high priority for clinical attention in discussions with current smokers, who should be informed of their continuing risk of lung cancer. Screening should not be viewed as an alternative to smoking cessation.”
(American Cancer Society: http://www.cancer.org/acs/groups/cid/documents/webcontent/acspc-039558-pdf.pdf))
“The American Cancer Society has thoroughly reviewed the subject of lung cancer screening and issued guidelines that are aimed at doctors and other health care providers:
Patients should be asked about their smoking history. Patients who meet ALL of the following criteria may be candidates for lung cancer screening:
- 55 to 74 years old
- In fairly good health (discussed further down)
- Have at least a 30 pack-year smoking history (Someone who smoked a pack of cigarettes per day for 30 years has a 30 pack-year smoking history, as does someone who smoked 2 packs a day for 10 years and then a pack a day for another 10 years.)
- Are either still smoking or have quit smoking within the last 15 years.
These criteria were based on what was used in the NLST.
Doctors should talk to these patients about the benefits, limitations, and potential harms of lung cancer screening. Screening should only be done at facilities that have the right type of CT scan and that have a great deal of experience in LDCT scans for lung cancer screening. The facility should also have a team of specialists that can provide the appropriate care and follow-up of patients with abnormal results on the scans.
Screening is meant to find cancer in people who do not have symptoms of the disease. People who already have symptoms that might be caused by lung cancer may need tests such as CT scans to find the underlying cause, which in some cases may be cancer. Still, this kind of testing is for diagnosis and is not the same as screening. Some of the possible symptoms of lung cancer that kept people out of the NLST were coughing up blood and weight loss without trying. To get the most potential benefit from screening, patients need to be in good health. For example, they need to be able to have surgery and other treatments to try to cure lung cancer if it is found. Patients who require home oxygen therapy most likely could not withstand having part of a lung removed, and so are not candidates for screening. Patients with other serious medical problems that would shorten their lives or keep them from having surgery may also not be able to benefit enough from screening for it to be worth the risks, and so should also not be screened. Metal implants in the chest (like pacemakers) or back (like rods in the spine) can interfere with x-rays and lead to poor quality CT images of the lungs. People with these types of implants were also kept out of the NLST, and so should not be screened with CT scans for lung cancer according to the ACS guidelines. People who have been treated for lung cancer often have follow-up tests, including CT scans to see if the cancer has come back or spread. This is called surveillance and is not the same as screening. (People with a prior history of lung cancer were not eligible for the NLST.)”
We note that the American College of Radiology commented on the American Cancer Society screening guidelines (described earlier).
“The American College of Radiology (ACR) acknowledges the importance of the new American Cancer Society recommendations for lung cancer screening using computed tomography (CT) scans. The ACR stresses that guidelines and practice standards are needed, and must be appropriately implemented, to ensure that patients nationwide have access to uniform, quality care and can expect a similar life-saving benefit from these exams as demonstrated in clinical trials.
Such guidelines must take into consideration the potential for false positives, incidental findings and other consequences that might negatively impact patients. The ACR is working on a practice guideline as well as ACR Appropriateness Criteria® based on thorough vetting of the peer reviewed literature.”
‘We continue to push forward in our work on standards regarding how and when these screening exams are conducted to help make sure that those who should be screened can do so regardless of where they live, but these standards need adequate time to be finalized to support a robust screening program that will provide the life-saving results that everyone wants,’ said Paul Ellenbogen, MD, FACR, chair of the ACR Board of Chancellors.”
The ACR supports the use of techniques shown to significantly reduce the number of people who die each year from lung cancer. Recent evidence, particularly results of the National Lung Cancer Screening Trial (NLST), has shown that CT lung cancer screening is appropriate when performed in the context of careful patient selection and follow-up.
However, barriers to widespread access to CT lung cancer screening remain — including acceptance of the cost-effectiveness of such a national screening program by Medicare and private insurers and associated coverage of these exams. While some insurers cover these exams, many may be waiting for the cost-effectiveness data from the NLST to be published later this year.”
6. Modeling
de Koning HJ, Meza R, Plevritis SK, ten Haaf K, Munshi VN, Jeon J, Erdogan SA, Kong CY, Han SS, van Rosmalen J, Choi SE, Pinsky PF, Berrington de Gonzalez A, Berg CD, Black WC, Tammemägi MC, Hazelton WD, Feuer EJ, McMahon PM. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med. 2014 Mar 4;160(5):311-20. doi: 10.7326/M13-2316. PMID: 24379002
de Koning and colleagues reported the results of simulation models of LDCT lung cancer screening scenarios “to estimate future harms and benefits of lung cancer screening and identify a set of possible efficient lung cancer screening policies by using 5 separately developed microsimulation models calibrated to the 2 largest randomized controlled trials on lung cancer screening.” Five simulation models [Erasmus Medical Center in Rotterdam, the Netherlands (model E); Fred Hutchinson Cancer Research Center in Seattle, Washington (model F); the Massachusetts General Hospital in Boston, Massachusetts (model M); Stanford University in Stanford, California (model S); and the University of Michigan in Ann Arbor, Michigan (model U)] were calibrated according to the NLST and the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO). In all models, 100 % adherence was assumed.
The investigators reported: “The most advantageous strategy was annual screening from ages 55 through 80 years for ever smokers with a smoking history of at least 30 pack-years and ex-smokers with less than 15 years since quitting. It would lead to 50 % (model ranges, 45 % to 54 %) of cases of cancer being detected at an early stage (stage I/II), 575 screenings examinations per lung cancer death averted, a 14 % (range, 8.2 % to 23.5 %) reduction in lung cancer mortality, 497 lung cancer deaths averted, and 5250 life-years gained per the 100 000-member cohort. Harms would include 67 550 false-positive test results, 910 biopsies or surgeries for benign lesions, and 190 over diagnosed cases of cancer (3.7 % of all cases of lung cancer [model ranges, 1.4 % to 8.3 %]).”
It is unclear why and how the PLCO (Oken, 2011), which evaluated chest x-ray screening, was used in model calibration. The simulation of LDCT screening appears to be based entirely on the NLST results and parameters since no other trial on LDCT was included. Sensitivity analysis of harms was specifically reported. Specific harms of follow-up were not listed.
7. Public Comments
The comments received on the proposed national coverage determination can be viewed in their entirety on the CMS Website at: http://www.cms.gov/medicare-coverage-database/details/nca-view-public-comments.aspx?NCAId=274.
During the initial 30-day comment period (2/10/2014 – 3/12/2014), CMS received 330 comments from various entities including providers, advocacy organizations, academic institutions, trade associations, and the general public. Of the comments received, 278 commenters advocated for coverage of lung cancer screening with LDCT, 27 supported coverage with conditions, 2 opposed, and 23 did not express a position.
During the 30-day comment period on the proposed NCD (11/10/2014 – 12/10/2014), CMS received 133 comments from various entities including professional medical societies, medical professionals and facilities, imaging centers, patient advocacy organizations, research consultants, device manufacturers, and the general public. Commenters were generally supportive of the proposed national coverage determination to expand Medicare coverage to include lung cancer screening as an additional preventive service.
A number of commenters supported coverage of lung cancer screening with LDCT noting that it is a positive step forward and provides for early detection of lung cancer in appropriate beneficiaries at high risk. Supportive commenters also believed that the proposed national coverage determination balances the risks, harms, and benefits of screening, while ensuring appropriate access to these services, and believed that lung cancer screening with LDCT requires careful implementation in centers of proven expertise in chest radiology, pulmonary thoracic surgery, and medical and radiation oncology, in a multidisciplinary and coordinated effort with registry management of findings and follow-up.
Some commenters expressed their support while also raising points for CMS to consider and suggested modifications to the proposed NCD. Some commenters did not express an opinion on the proposed NCD. Commenters addressed additional risk factors for lung cancer and conditions like asbestosis and radon exposure. A few commenters were opposed to coverage for these services, with some opining that if individuals choose to smoke that Medicare funds should not be used for screening and treatment of resulting conditions. Others suggested that Medicare should consider coverage for other screening modalities instead of lung cancer screening with LDCT.
Commenters provided feedback on, and suggested modifications to, the proposed coverage requirements for beneficiaries, radiologists, and imaging centers. Our responses to the public comments are addressed below.
Beneficiary Eligibility Criteria
Comment:
Commenters suggested that CMS reassess the proposed age criteria of 55-74, specifically to expand coverage for beneficiaries up to age 80, with some commenters opining that the median age of diagnosis of 70 suggests that those older than 74 would also benefit from screening. Many commenters believed that the age criteria should align with the USPSTF recommendation, with a few commenters opining that CMS’ proposed national coverage determination would violate the equal protection clause of the Fourteenth Amendment of the United States Constitution since private insurers cover preventive services consistent with USPSTF recommendations for these services. Some commenters recommended expanding coverage based on modeling studies. Other commenters suggested that a beneficiary’s overall health status is a better indicator of how a beneficiary will handle cancer treatments rather than a specific age range and suggested eliminating the age criteria in its entirety.
Response:
We do not agree with the commenters that suggest that the proposed age range and coverage requirements violate the equal protection clause of the Fourteenth Amendment. By its terms, that amendment applies to States and not to the Federal government. Additionally, the statute governing “additional preventive services” for the Medicare program, §1861(ddd) of the Social Security Act, is not the same as the statute governing coverage of preventive health services for group health plans under 42 U.S.C. § 300gg-13. The statute governing coverage of preventive services for group health plans ensures coverage of certain immunizations and additional preventive services for infants, children, adolescents and women that are not included in title XVIII of the Social Security Act. The two statutes are not identical and the commenters are comparing two groups of patients who are not similarly situated.
Although it is true that both statutes recognize the importance of items and services that have received grade A or B recommendations by the USPSTF, the Medicare statute also enables the Secretary to consider whether the service is “reasonable and necessary for the prevention or early detection of an illness or disability” and “appropriate for individuals entitled to benefits under part A or enrolled in part B” before adding a new preventive service under part B. We believe that these two aspects of Section 1861(ddd) of the Social Security Act provide the authority to treat Medicare beneficiaries differently.
As discussed in the analysis, our age range was set using the enrollment age criteria of the NLST. As the study participants continued with the three annual screenings, benefit and harms data were recorded on participants up to and including the age of 77 years. Data on these patients were analyzed along with the other trial participants to demonstrate overall benefits. In the age specific analysis (Pinsky, 2014), no statistically significant increase in harms was reported. Therefore, we are modifying the beneficiary eligibility criteria to include beneficiaries 55 to 77 years of age.
There is no empirical data available for adults aged 78-80 years nor is there adequate evidence to cover LDCT screening for individuals beyond 55 – 77 years of age. Data from well-done randomized controlled trials provide the strongest evidence. We believe data from modeling is inadequate to ensure that the service would be “reasonable and necessary” or “appropriate” for Medicare beneficiaries, for purposes of §1861(ddd) of the Social Security Act.
Comment:
Several commenters requested that CMS modify the asymptomatic criterion. Commenters believed that to require the beneficiary show no signs or symptoms of lung disease would inappropriately limit coverage for this benefit. One commenter suggested allowing coverage for all beneficiaries, regardless of whether they had signs or symptoms of lung disease.
Response:
In response to the commenters’ concerns, we are modifying this beneficiary eligibility criterion to state that a beneficiary must be “asymptomatic (no signs or symptoms of lung cancer)”, consistent with the evidence reviewed and comments received.
Comment:
A few commenters suggested that Medicare cover NCCN Group 2 (ages 50-54, 20-pack year smoking history, and one additional risk factor for lung cancer) under coverage with evidence development. Commenters opined that individuals in NCCN Group 2 are equivalent to individuals that would fall under NCCN Group 1. Other commenters asked for expanded coverage to those who have quit smoking more than 15 years ago.
Response:
The NLST was the only trial (of several trials and observational studies over the past decade) to show benefits of lung cancer screening with LDCT. The NLST provided the evidence to determine that this service is “reasonable and necessary” and “appropriate” for Medicare beneficiaries. We did not find evidence of improvements in health outcomes in other populations, such as those suggested by the commenters (for instance, individuals that have a smoking cessation history greater than 15 years and individuals with a 20-pack year smoking history). We will continue to closely monitor ongoing trials, which we believe will improve the evidence base, and will consider modifying coverage in the future as appropriate.
Counseling and Shared Decision Making Visit/Written Orders
Comment:
Commenters generally supported the requirement that for the initial LDCT lung cancer screening, beneficiaries obtain a written order from a counseling and shared decision making visit. Others suggested that requiring a separate visit was difficult to incorporate into normal physician practice, and that mandating a face-to-face visit would limit access to screening services for beneficiaries in rural areas. One commenter questioned the need for the shared decision making visit if the written order was properly documented. Commenters also asked whether the counseling and shared decision making visit would be required for individuals that started lung cancer screening prior to becoming Medicare beneficiaries.
Response:
We believe that a counseling and shared decision making visit that addresses the benefits and harms of screening is supported by the evidence and is essential for ensuring that appropriate eligible beneficiaries receive these initial services with full knowledge of the risks, benefits, and commitment necessary to receive the most benefit from a lung cancer screening program. Among other things, there is the potential for significant harms in starting a lung cancer screening program, including the risk for false-positive results leading to additional tests and treatments that may be more harmful. The goal of shared decision making is not merely to furnish a written order for such services, but that both the practitioner and the beneficiary are armed with a better understanding of the relevant risk factors, and are engaged with shared responsibility regarding the decision to proceed or not proceed with a lung cancer screening program. We believe that the initial counseling and shared decision making visit supports identification of individuals that would most benefit from a lung cancer screening program.
Comment:
Commenters requested clarification about who could furnish the initial counseling and shared decision making visit, with others opining that nurses, psychologists, and imaging facilities should also be eligible to furnish these services.
Response:
We believe that formal shared decision making between the physician or qualified non-physician practitioner and the beneficiary is an important and essential component to an effective lung cancer screening program. We leave it to the discretion of the physician or qualified non-physician practitioner to determine whether other medical professionals should also participate in the visit based on a particular beneficiary’s needs. Therefore, we are maintaining the requirement that the counseling and shared decision making visit must be furnished by a physician or qualified non-physician practitioner.
Comment:
A few commenters asked whether standardized instruments existed that could be used during the initial counseling and shared decision making visit and whether such instruments would need to be approved by CMS.
Response:
We understand that specific decision aids for lung cancer screening are being developed and will become more readily available as screening is more broadly adopted. While we are not requiring the use of a specific instrument, eligible practitioners may select from various available decision aids designed for this purpose and recognized by national professional medical organizations. For instance, the National Institutes of Health’s National Cancer Institute has developed a decision aid/tool, available at: http://www.cancer.gov/newscenter/qa/2002/NLSTstudyGuidePatientsPhysicians.
Comment:
For subsequent screenings, some commenters disagreed with the requirement that the beneficiary must receive a written order. Other commenters requested clarification about how a written order for subsequent screenings should be obtained.
Response:
We agree that for subsequent screenings, a separate counseling and shared decision making visit (as required for the initial screening) would be overly burdensome. However, we believe that in order to balance the benefits, harms, and complex nature of this service, the beneficiary needs to obtain a written order for subsequent screenings to support continued shared decision making in light of the beneficiary’s changing health status, particularly as one ages. For example, the Medicare-covered annual wellness visit (AWV) may be one option for obtaining such an order. The AWV includes elements for the furnishing of appropriate referrals and orders for preventive services.
Therefore, we are maintaining the criterion that a beneficiary must receive a written order for subsequent screenings during any appropriate visit with a physician or qualified non-physician practitioner. While we are not requiring a separate counseling and shared decision making visit for subsequent screenings, we are modifying this criterion to note that if a physician or qualified non-physician practitioner chooses to furnish a counseling and shared decision making visit for subsequent screenings, the visit must include all of the components required for an initial counseling and shared decision making visit as defined in this NCD.
Reading Radiologist Eligibility Criteria
Comment:
Commenters suggested that CMS modify the proposed requirements for radiologists eligible to perform lung cancer screening to add board eligibility in addition to proposed board certification. Another commenter suggested that CMS remove the requirement for board certification.
Response:
In response to the comments received, we are modifying the criterion to indicate that reading radiologists eligible to furnish these services must be board certified or board eligible with the American Board of Radiology or equivalent organization. These modifications are consistent with the evidence reviewed, including the NLST, where the reading radiologists were American Board of Radiology certified or board-eligible or had equivalent qualifications as defined by the American College of Radiology Practice Guidelines.
Radiology Imaging Facility Eligibility Criteria
Comment:
Commenters suggested modifications to the coverage requirements for radiology imaging facilities. Some commenters asked that CMS allow screening to be performed by an accredited provider of chest CT with training and experience in LDCT lung cancer screening. Other commenters requested clarification on how the criteria to be an advanced diagnostic imaging center would be met. One commenter requested that CMS use a professional medical society’s lung cancer screening center certification program as a template for facility certification. Another commenter noted that all centers should meet the same eligibility requirements.
One commenter suggested that screening should only be covered in imaging facilities that participated in lung cancer screening trials since the commenter opined that the value of lung cancer screening varies markedly depending on how it is implemented. A few commenters were concerned that without quality requirements, patients could be at risk for over-exposure to radiation and/or have poor quality exams, resulting in additional harm and missed cancers. Other commenters suggested other facility types may be appropriate to perform these services, such as hospital imaging departments.
Commenters also opined that requiring past participation in the NLST or advanced diagnostic imaging center accreditation with training and experience in LDCT lung cancer screening would place an undue burden on patients and rural facilities. Some commenters recommended that CMS acknowledge professional society accreditation or participation in other studies besides the NLST for purposes of meeting the eligibility criteria, while also ensuring quality standards.
Response:
We appreciate the commenters’ extensive feedback regarding the proposed imaging facility eligibility criteria. While we agree with some of the commenters that requiring past participation in the NLST or advanced diagnostic imaging center accreditation with experience in LDCT lung cancer screening would place an undue burden on imaging facilities, we also agree with the commenters who expressed concern that without quality requirements, beneficiaries could be exposed to additional harms. Included in CMS’ review of the evidence were evidence-based recommendations on the necessary components of a high quality lung cancer screening program. As reported by Mazzone and colleagues (2014), necessary components include, among other things, the establishment of a lung nodule identification, classification, and reporting system; and integration of smoking cessation interventions into a lung cancer screening program.
Based on the evidence reviewed, including the public comments received, and to ensure appropriate access to these services, we are modifying the imaging facility eligibility criteria by removing the requirement for either past participation in lung cancer screening trials or advanced diagnostic imaging accreditation with training and experience in LDCT lung cancer screening. We are requiring that imaging facilities utilize standardized lung nodule identification, classification, and reporting systems, and make available smoking cessation interventions for current smokers, based on the evidence reviewed. We believe these modifications strike an appropriate balance between maintaining appropriate and high quality access to these services, while reducing burden.
Comment:
Regarding the effective radiation dose, commenters suggested various technical revisions including that the radiation dose should be as low as possible, and that CMS should allow for adjustments based on the size of the individual. Some commenters opined that milligray (mGy) is a more common measurement than millisievert (mSv) for expressing volumetric dose.
Response:
We are revising the imaging facility eligibility criteria in light of the public comments. The proposed radiation dose was developed to align with the NLST. The NLST research team (2011) reported that the acquisition variables were chosen to reduce exposure to an average effective dose of 1.5 mSv.” Based on the comments received and the evidence reviewed (including recently published evidence-based multi-society, multi-disciplinary professional guidelines), we are modifying the imaging facility criteria to state that an eligible facility must be one that performs LDCT with volumetric CT dose index (CTDIvol) of ≤ 3.0 mGy (milligray) for standard size patients (defined to be 5’ 7” tall; and approximately 155 pounds) with appropriate reductions in CTDIvol for smaller patients and appropriate increases in CTDIvol for larger patients.
Registry Requirements and Data Elements
Comment:
Some commenters expressed concern about the feasibility of tracking and submitting registry data related to diagnostic follow-up, cancer incidence, and lung-cancer/all-cause mortality. Commenters were concerned that tracking diagnostic procedures and outcomes may be logistically difficult since diagnostic follow-up may not necessarily be carried out at the center performing the LDCT screening. A few commenters suggested that a registry is not needed for these services and would limit access to screening (particularly in small, non-academic facilities). Others indicated that the number of data elements proposed is overly burdensome. Commenters’ suggested alternatives included limiting registry data elements to those items that could be ascertained at the time of the screen or shortly thereafter (screening results). Some commenters suggested adding additional data elements to the registry that capture other risks for lung cancer. One commenter suggested that CMS review and reference current consensus guidelines from various professional societies as a reporting mechanism. One commenter requested information about existing registries that would meet CMS requirements, while another asked for clarification about the objectives, planned uses of the registry data, requirements for registry administration, and CMS solicitation and approval of registries.
Response:
The primary purpose for requiring the submission of data to the registry is to document compliance with the coverage criteria that are not evidenced on the health care claim. Furthermore, based on the public comments and the evidence reviewed, we strongly believe that the registry will serve as an aid to those seeking to study the clinical benefits of this screening. The registry and the other criteria required in this NCD are supported by the evidence reviewed, including the NLST. The registry will help ensure that only eligible beneficiaries will receive this screening service since only beneficiaries that meet the eligibility requirements will benefit from such screening.
Furthermore, we recognize the impact of this criterion for imaging facilities. We will only require production of the minimum number of data elements to carry out this payment function in an effort to reduce burdens. Therefore, we are modifying the data registry elements, based on our review of the evidence and feedback received from commenters. As amended, the data elements are limited to those required to determine whether an individual has met the coverage criteria for the LDCT lung cancer screening service, that is, whether their receipt of the service was “reasonable and necessary” and “appropriate.” Data collected and submitted to a CMS-approved national registry must include, at minimum, all of the following elements:
| Data Type |
Minimum Required Data Elements |
| Facility |
Identifier |
| Radiologist (reading) |
National Provider Identifier (NPI) |
| Patient |
Identifier |
| Ordering Practitioner |
National Provider Identifier (NPI) |
| CT scanner |
Manufacturer, Model |
| Indication |
Lung cancer LDCT screening – absence of signs or symptoms of lung cancer |
| System |
Lung nodule identification, classification and reporting system |
| Smoking history |
Current status (current, former, never),
If former smoker, years since quitting,
Pack-years as reported by the ordering practitioner,
For current smokers, smoking cessation interventions available. |
| Effective radiation dose |
CT Dose Index (CTDIvol) |
| Screening |
Screen date
Initial Screen or
Subsequent Screen |
Additionally, national registries are strongly encouraged to collect data on lung nodules (for example: clinically significant non-lung cancer findings, the number and types of nodules, and size and location of each nodule), subsequent diagnostic testing, adverse events, and intermediate and long term health outcomes, in order to inform practices and policymakers about the ability to implement a LDCT lung cancer screening program broadly in multiple settings across the country, and achieve positive outcomes, consistent with the NLST. We recognize that these other data elements are extremely important to establishing the benefit of these screening services and improvement in health outcomes. We strongly encourage submission of such data elements to registries in addition to the minimum elements required under this NCD. These data will not only verify that screening leads to improved health outcomes for the Medicare population, but will also serve as the basis to refine and improve screening in practice, and serve the quality improvement purposes of screening facilities. We believe that multi-society stakeholders are in the best position to determine the appropriate data elements for reaching these goals, and to adjust the particular elements over time.
All CMS-approved registries must have the capacity and capability to collect data from any Medicare-eligible imaging facility/department that furnishes lung cancer screening with LDCT, with a catchment area that includes all 50 States, United States Territories, and the District of Columbia. CMS will evaluate each entity interested in participating as a CMS-approved registry to determine if they are capable of meeting the registry and data collection requirements outlined in this national coverage determination, including:
- Establishment of a steering committee and a governance board for oversight of the registry;
- Registry management plan, including identification of key personnel;
- Operational plan and framework that describes mechanisms for collection and submission of data from imaging facilities to the registry;
- Registry catchment area;
- Mechanisms for the submission of registry data to CMS electronically;
- Mechanisms to collect information (e.g.; HICN) in order to permit linkage of registry data with external databases (e.g. Medicare claims data sets);
- Description of data management and data quality review methods, including validation;
- Use of CMS-approved standardized data dictionary;
- Mechanisms for submitting a list of facilities participating in the registry to CMS; and
- Quality assurance plan.
To apply to function as a CMS-approved registry, interested entities must submit a letter of interest along with detailed supporting information about how the interested entity is able to meet the requirements outlined in this national coverage determination to the following address or via email to caginquiries@cms.hhs.gov.
Centers for Medicare & Medicaid Services
Center for Clinical Standards and Quality
Director, Coverage and Analysis Group
ATTN: Lung Cancer LDCT Screening
Mail Stop: S3-02-01
7500 Security Blvd.
Baltimore, MD 21244
Information regarding CMS-approved registries will be posted on the CMS website.
Additional Comments
Comment:
One commenter asked that CMS ensure that lung cancer screening is performed as a part of a dedicated, multi-disciplinary program to image, diagnose and manage high risk patients and opined that lung cancer screening is only one part of the paradigm of management of high risk patients in order to achieve positive results with lung cancer screening.
Response:
We have set coverage criteria to implement lung cancer screening with LDCT in a responsible manner as part of a lung cancer screening program, in order to ensure positive outcomes of this screening service in the Medicare population. These coverage criteria are consistent with the evidence reviewed including the NLST and information about “implementation of a lung cancer screening program” from the USPSTF (Moyer 2014).
Comment:
One commenter expressed concern about possible over-diagnosis of lung cancer in the screened population and the effect that screening would have on post-lobectomy survival rates.
Response:
We appreciate these commenters’ concerns. They also reflect the concerns raised by the MEDCAC. We acknowledge that there is the potential for significant harms in starting a lung cancer screening program, including the risk for false-positive results leading to additional tests and treatments that may be more harmful. There are potential benefits of lung cancer screening with LDCT if implemented carefully. We are establishing specific beneficiary, provider and imaging facility eligibility requirements, along with inclusion of a counseling and shared decision making visit to ensure that the benefits of screening outweigh harms for the Medicare population.
Comment:
A few commenters were opposed to Medicare coverage of lung cancer screening with LDCT, with some commenters suggesting that other screening methods, such as breath tests, should be considered for coverage instead of lung cancer screening with LDCT. A few commenters believe that Medicare funds should not be used for lung cancer screening in individuals who have chosen to smoke. A few commenters asked about other risk factors for lung cancer such as radon or asbestos exposure.
Response:
We appreciate the commenters’ concerns since lung cancer is the leading cause of cancer deaths in the United States. At this time, there are no other proven screening tests. The USPSTF grade B recommendation was limited to patients with a history of smoking and the evidence in the NLST focused on heavy smoking. Therefore, we consider these suggestions to be outside the scope of this national coverage analysis. In order to consider adding screening for lung cancer for additional indications or conditions, there would need to be a specific recommendation from the USPSTF along with evidence concerning the other factors identified in §1861(ddd) of the Social Security Act and regulations at 42 C.F.R. § 410.64.
We believe that this national coverage determination implements lung cancer screening with LDCT in a responsible manner. CMS will continue to monitor the evidence as stewards of the Medicare program. We strongly encourage Medicare beneficiaries to stop smoking since smoking is a major risk factor for development of lung cancer.
Comment:
A few commenters requested information about coding, reimbursement, and Medicare-covered tobacco cessation counseling services.
Response:
While coding and reimbursement are outside the scope of this national coverage analysis, we plan to develop appropriate educational materials, including a Medicare Learning Network article. Information regarding Medicare coverage of tobacco cessation counseling services can be found in Pub. 100-03, Medicare National Coverage Determinations Manual, Chapter 1, Sections 210.4 and 210.4.1.
Comment:
One commenter believed that CMS did not have authority to designate eligibility criteria for facilities and radiologists because medical licensure is determined by State governments.
Response:
While we agree that medical licensure is primarily a State responsibility, Congress has frequently established specific conditions of coverage or payment for particular services under the Medicare program. Congress has expressly authorized CMS to add coverage for additional preventive services under certain conditions as set forth in §1861(ddd) of the Act, when those services are found to be appropriate for individuals entitled to benefits under part A or enrolled under part B, as determined via the NCD process. As part of this appropriateness determination, it is reasonable to consider the qualification of the radiologists and facilities that may be furnishing this new service. It would be difficult, if not impossible, to expand coverage for a new preventive service without addressing the qualifications of the providers and suppliers who are to be paid for furnishing this care under a national program.
We are finalizing eligibility criteria for radiologists and imaging facilities for lung cancer screening with modifications based on the evidence reviewed, including public comments, in light of some of the added risks of this particular procedure (for example, exposure to radiation even at low doses creates a patient safety concern). Using the NCD process, we have asked for and received public comments about those criteria. We have made some changes to those criteria as a result of the public comments. We believe our actions are consistent with our authority under the Medicare statute and do not interfere with State licensing determinations.
VIII. Analysis
National coverage determinations (NCDs) are determinations by the Secretary with respect to whether or not a particular item or service is covered nationally under title XVIII of the Social Security Act (§ 1869(f)(1)(B)). In order to be covered by Medicare, an item or service must fall within one or more benefit categories contained within Part A or Part B, and must not be otherwise excluded from coverage. Since January 1, 2009, CMS is authorized to cover "additional preventive services" (see Section III above) if certain statutory requirements are met as provided under § 1861(ddd) of the Social Security Act. Regulations at 42 C.F.R. § 410.64 provide:
(a) Medicare Part B pays for additional preventive services not described in paragraph (1) or (3) of the definition of “preventive services” under §410.2, that identify medical conditions or risk factors for individuals if the Secretary determines through the national coverage determination process (as defined in section 1869(f)(1)(B) of the Act) that these services are all of the following:
(1) Reasonable and necessary for the prevention or early detection of illness or disability.
(2) Recommended with a grade of A or B by the United States Preventive Services Task Force.
(3) Appropriate for individuals entitled to benefits under part A or enrolled under Part B.
(b) In making determinations under paragraph (a) of this section regarding the coverage of a new preventive service, the Secretary may conduct an assessment of the relation between predicted outcomes and the expenditures for such services and may take into account the results of such an assessment in making such national coverage determinations.
Question 1: Is the evidence sufficient to determine that screening for lung cancer with low dose computed tomography is recommended with a grade of A or B by the United States Preventive Services Task Force?
In 2014, Moyer and colleagues, on behalf of the USPSTF, reported: “The USPSTF recommends annual screening for lung cancer with low-dose computed tomography in adults aged 55 to 80 years who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery. (B recommendation)” (Moyer, 2014). The evidence is sufficient to conclude that lung cancer screening with LDCT is recommended with a grade B by the USPSTF.
Prior to the current recommendation, the USPSTF evaluated lung cancer screening in 1996 (grade D) and in 2004 (grade I – consistent with current I statement) based upon 6 observational studies (Humphrey, 2004). The change in recommendation was based upon four trials (DANTE, DLSCT, MILD, NLST) (Humphrey, 2011). Net benefit of screening was supported primarily by the NLST (for individuals aged 55 to 74 years who have &#≥ 30 pack-year smoking histories and current smokers or have quit in the past 15 years). Extension of the upper age beyond NLST data was based upon modeling only, with no empirical data.
In the 2014 considerations, the USPSTF specifically noted recommendations for:
“Implementation of a Lung Cancer Screening Program
Screening Eligibility, Screening Intervals, and Starting and Stopping Ages
The NLST, the largest RCT to date with more than 50,000 patients, enrolled participants aged 55 to 74 years at the time of randomization who had a tobacco use history of at least 30 pack-years and were current smokers or had quit within the past 15 years. The USPSTF recommends extending the program used in the NLST through age 80 years. Screening should be discontinued once the person has not smoked for 15 years.
The NLST enrolled generally healthy persons, and the findings may not accurately reflect the balance of benefits and harms in those with comorbid conditions. The USPSTF recommends discontinuing screening if a person develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery.
Clinicians will encounter patients who are interested in screening but do not meet the criteria of high risk for lung cancer as described previously. The balance of benefits and harms of screening may be unfavorable in these lower-risk patients. Current evidence is lacking on the net benefit of expanding LDCT screening to include lower-risk patients. It is important that persons who are at lower risk for lung cancer be aware of the potential harms of screening. Future improvements in risk assessment tools will help clinicians better individualize patients' risks.
Smoking Cessation Counseling
All persons enrolled in a screening program should receive smoking cessation interventions. To be consistent with the USPSTF recommendation on counseling and interventions to prevent tobacco use and tobacco-caused disease, persons who are referred to a lung cancer screening program through primary care should receive these interventions before referral. Because many persons may enter screening through pathways besides referral from primary care, the USPSTF encourages incorporating such interventions into the screening program.
Shared Decision Making
Shared decision making is important for persons within the population for whom screening is recommended. The benefit of screening varies with risk because persons who are at higher risk because of smoking history or other risk factors are more likely to benefit. Screening cannot prevent most lung cancer deaths, and smoking cessation remains essential. Lung cancer screening has substantial harms, most notably the risk for false-positive results and incidental findings that lead to a cascade of testing and treatment that may result in more harms, including the anxiety of living with a lesion that may be cancer. Overdiagnosis of lung cancer and the risks of radiation are real harms, although their magnitude is uncertain. The decision to begin screening should be the result of a thorough discussion of the possible benefits, limitations, and known and uncertain harms.
Standardization of LDCT Screening and Follow-Up of Abnormal Findings
The evidence for the effectiveness of screening for lung cancer with LDCT comes from RCTs done in large academic medical centers with expertise in using LDCT and diagnosing and managing abnormal lung lesions. Clinical settings that have high rates of diagnostic accuracy using LDCT, appropriate follow-up protocols for positive results, and clear criteria for doing invasive procedures are more likely to duplicate the results found in trials. The USPSTF supports adherence to quality standards for LDCT and establishing protocols to follow up abnormal results, such as those proposed by the National Comprehensive Cancer Network (NCCN). A mechanism should be implemented to ensure adherence to these standards.
In the context of substantial uncertainty about how best to manage individual lesions, as well as the magnitude of some of the harms of screening, the USPSTF encourages the development of a registry to ensure that appropriate data are collected from screening programs to foster continuous improvement over time. The registry should also compile data on incidental findings and the testing and interventions that occur as a result of these findings.”
Question 2: Is the evidence sufficient to determine that screening for lung cancer with low dose computed tomography is reasonable and necessary for the prevention or early detection of illness or disability?
Since 2004, four trials (DANTE, DLSCT, MILD, NLST) evaluated lung cancer screening using LDCT. One large trial (NLST, 2011; n = 53,454) showed three annual LDCT screenings of individuals aged 55 to 74 years, who have &#≥ 30 pack-year smoking history, and are current smokers or have quit in the past 15 years, reduced lung cancer mortality. Two smaller trials (DANTE, 2009, n = 2472; DLSCT, 2012, n = 4104) did not find a significant difference in lung cancer mortality between LDCT screening compared to control (no screening). One trial compared annual to biennial screening and found no significant difference in lung cancer mortality (MILD, 2012, n = 4099).
The NLST is the only trial to demonstrate benefit of lung cancer screening with LDCT. It was a well conducted, large multicenter trial performed at 33 centers with trained and approved radiologists and follow-up evaluation at experienced centers. Investigators noted: “the NLST was conducted at a variety of medical institutions, many of which are recognized for their expertise in radiology and in the diagnosis and treatment of cancer. It is possible that community facilities will be less prepared to undertake screening programs and the medical care that must be associated with them.”
DANTE and DLSCT evaluated participants who were similar in age of those studied in the NLST, but had a smaller smoking history &#≥ 20 pack-year and compared to no screening. The MILD trial had methodologic issues which reduced study quality in comparison to the other included trials. In past trials, the benefits, mainly in lung cancer mortality, have been difficult to demonstrate due to a number of potential factors including patient selection, along with diagnosis and treatment following positive findings.
The NLST demonstrated benefit by enrolling a large number of high exposure patients (smoking history) to be followed for several years to detect a significant decrease (247 deaths from lung cancer per 100,000 person-years in the low-dose CT group and 309 deaths per 100,000 person-years in the radiography group; number needed to screen (NNS) to prevent one lung cancer death = 320). The trial was ended early after an interim analysis. The decision to compare to screening with chest radiology (Church, 1990) was made before the PLCO trial was completed that showed chest radiology did not reduce lung cancer mortality (Oken, 2011); however, the radiology group was exposed to harms that may not have occurred in a no screening control, potentially enhancing the relative benefits and the likelihood of a positive trial result. The harms of LDCT relate to the CT scan itself and the follow-up diagnostic tests or interventions (adverse events from bronchoscopies and biopsies), and patient psychosocial consequences, and have been recognized for many years as noted in past USPSTF reviews. For example, death within 60 days after most invasive diagnostic procedures was twice as high in the radiology group compared to the LDCT group (2.1 % versus 1.0 %; NLST, 2011), which with a large sample size may result in a meaningful difference. NLST investigators wrote: “one of the most important factors determining the success of screening will be the mortality associated with surgical resection, which was much lower in the NLST than has been reported previously in the general U.S. population (1 % vs. 4 %).” If this is not maintained with broad implementation of screening, the screening benefits may not be realized. A better understanding of why patients screened with LDCT had lower mortality from invasive procedures is needed. Questions such as whether the state of disease, patient, physician, or bias of either the physician/practitioner or patient may need to be considered.
While age inclusion criteria were comparable among trials, the majority of participants were in the 50-64 year age range. In the NLST, 26.6 percent (7110/26,722) of the individuals in the LDCT screening group were 65 years of age or older. Pinsky et al. (2014) noted: “NLST participants aged 65 years or older had a higher rate of false-positive screening results than those younger than 65 years by a higher cancer prevalence and PPV” [positive predictive value].
The NLST enrolled patients aged 55 to 74 and followed each of these participants at least 3 years. Therefore, we have data up to age 77 for some participants that entered the NLST at age 74. We had proposed an age range based on the NLST inclusion criteria but will extend the upper age to 77 years based on actual trial data. There are no trial data or evidence on adults over 77 years. Modeling was used to extend the age limit for the USPSTF recommendation. However, results from modeling vary widely based on the assumptions used in the modeling. de Koning et al. specifically reported that “models assumed 100 % adherence with screening,” an unrealistic long term assumption for real world screening that even the NLST could not achieve (95 % adherence with three annual screens in LDCT group of NLST). The authors also noted that “extrapolations beyond those trials’ [NLST, PLCO] time horizons, screening intervals, and eligibility criteria introduce uncertainty” (de Koning, 2014). It is difficult to interpret modeling of benefits and harms since models may be limited in consideration of older patients if inputs are based solely on data from younger populations. Patz et al. (2014) noted: “[a]s with any model, one should be cautious in extrapolating much beyond the data on which the model was based, which in this case are 3 annual screens and a total of approximately 7 years of follow-up.”
It is recognized that the number and severity of comorbidities increases with age (Medicare Current Beneficiary Survey, 2011). Comorbidities may then influence the ability to tolerate invasive diagnostic procedures (e.g., risk for adverse events from bronchoscopy; Hehn, 2003) and may reduce the response of cancer treatments in older adults with multiple comorbidities. Wang and colleagues (2012) noted: “[c]omorbidity can have an impact on care for patients with NSCLC [non–small-cell lung cancer] in a variety of ways. First, patients with comorbidity are more likely to experience treatment toxicity, and treatments may exacerbate underlying comorbidity. Second, comorbidity decreases the likelihood of completing treatment. In a large trial (Frasci et al., 2000) patients with advanced NSCLC who had Charlson comorbidity index (CCI) scores &#≥ 2 were more likely to discontinue chemotherapy. Third, significant comorbidities can limit life expectancy, particularly in earlier-stage cancers, decreasing the potential survival benefit of cancer treatment.” Given these concerns without actual trial data to show that the benefits outweigh the harms, and the harms (e.g., mortality and morbidity from invasive procedures as biopsies) may be significant, the evidence is insufficient to determine that screening is reasonable and necessary for beneficiaries 78 years of age and older.
Smoking history is an important variable to consider since higher exposure increases risk; however, it is a difficult variable to accurately ascertain in general clinical practice due to recall or approximations since it is typically self-reported. A specific formal determination of smoking history is needed. Lung cancer screening outcomes may very well hinge on the initial determination. The NLST included individuals with &#≥ 30 pack-year (mean = 56) smoking history while DANTE and DLSCT enrolled individuals with &#≥ 20 pack-year smoking history. Over-diagnosis, the detection of indolent tumors that otherwise may not cause clinical symptoms [“more than 18 % of all lung cancers detected by LDCT in the NLST seem to be indolent” (Patz, 2014)], also influences the balance of benefits and harms of screening. The false positive rate of LDCT positive screens was very high in all three trials (DANTE, DLSCT, and NLST).
| |
Age
(inclusion criterion) |
Smoking history
(inclusion criterion) |
Positive result LDCT
(false positive rate) |
| DANTE; n = 2472
(Infante, 2009) |
mean age = 64.6;
(60-74 years) |
mean = 47 pack-years;
(&#≥ 20 pack-years) |
non-calcified nodule &#≥ 10 mm, etc; (291/351, 83%) |
| DLSCT; n = 4104
(Saghir, 2012) |
91% 50-64 years;
(50-70 years) |
mean = 36 pack-years;
(&#≥ 20 pack-years) |
nodule &#≥ 5 mm; (542/611, 89%) |
| NLST; n = 53454
(NLST, 2011) |
73% 55-64 years;
(55-74 years) |
mean = 56 pack-years;
(&#≥ 30 pack-years) |
non-calcified nodule &#≥ 4 mm; (17497/18146, 96 %) |
While progress has been made in limiting harms, especially radiation exposure, per screening scan, which was limited to an average of 1.5 mSv in the NLST, many individuals have follow-up chest CTs (LDCT or standard diagnostic CT in 49.8 percent in NLST LDCT group) or PET-CT scans (8.3 percent in NLST LDCT group; radiation dose of typical PET is greater than or equal to standard diagnostic CT). Accordingly, the low dose designation may be misleading in the sense that it may not reflect the total radiation exposure from screening for some individuals.
Women comprised 45 percent of the study population in DLSCT and 41 percent in NLST. Gender was borderline as an interaction in NLST (p = 0.08; Pinsky, 2013). DANTE enrolled only men but gender alone is unlikely to account for all of the observed differences between the trials. Other patient and provider characteristics, nodule size and evaluation, sample size and control group interventions were likely factors. The high LDCT screening false positive rate for lung cancer in these trials and the subsequent need for follow-up of small lesions are concerning. It is difficult to estimate the full extent that additional testing at community centers, and the false positive rate, will have in the Medicare population, along with the potential harms of subsequent evaluation in beneficiaries with advanced age and comorbidities.
Overall, based primarily on the results of the NLST, we find that the evidence is sufficient to determine that screening for lung cancer with LDCT is reasonable and necessary for the prevention or early detection of illness or disability for the exact population studied in the NLST. This recommended screening population is consistent with recommendations and guidelines from major organizations such as the American Cancer Society, American Lung Association, and the American College of Chest Physicians. The American College of Radiology recommends the broader group, similar to the USPSTF recommendation while the American Academy of Family Physicians found insufficient evidence to recommend for or against screening.
There are substantial concerns that the benefits demonstrated in NLST may not be replicated with broad screening in the Medicare population. The National Cancer Institute which sponsored the NLST noted: “Questions will still remain about whether the balance of benefits and harms found in a study conducted by centres that have strong expertise in screening can be maintained in less regulated and less quality-controlled settings in the general community” (Kramer, 2011). While recognizing the importance of the NLST, the MEDCAC reinforced these concerns according to the committee’s low confidence (2.22 out of 5 scale) that the benefits outweigh the harms of lung cancer screening with LDCT in the Medicare population (http://www.cms.gov/medicare-coverage-database/details/medcac-meeting-details.aspx?MEDCACId=68)
Marshall et al. (2013) noted: “[g]eneralization of findings from tightly controlled trial situations to large-scale mass screening programs require uniform standards and high quality control in order to be able to accurately track and assess nodules over time. Lung cancer screening is more than simple provision of a CT service; it is as a long-term commitment requiring extensive infrastructure to allow for invitation and recruitment; quality improvement; workforce/facility capacity for screening, diagnosis and treatment; health professional training; participant information and support. On-going evaluation and monitoring of the program is essential to ensure high standards of care are met and delivered in a consistent and acceptable way.”
Comments submitted to the USPSTF expressed similar concerns. The USPSTF responded by adding a section on implementation “emphasizing the need for monitoring this implementation, quality assurance in diagnostic imaging, and appropriate follow-up to replicate the benefits observed in the NLST in the general population.” This programmatic approach and implementation framework provide the foundation for our decision and enables LDCT lung cancer screening to be reasonable and necessary.
Since the NLST was the only trial of several trials and observational studies over the past decade to show benefits, there is no evidence of improvements in health outcomes from lung cancer screening using LDCT in other populations. The results of ongoing trials in Europe will help solidify the evidence base (Field, 2013). There is no evidence on LDCT lung cancer screening in adults over 75 years of age since no trial has included this subgroup. There is no evidence on screening with more than 3 annual LDCT scans. While modeling, if performed properly with evidence based assumptions, may generate interesting hypotheses, direct evidence from actual studies designed to test hypotheses
are still needed. Sox (2014) recently wrote: “No one wants to repeat the experience with prostate cancer screening, which became a de facto standard of practice long before evidence from randomized trials signaled caution. The era of lung cancer screening begins with randomized trial evidence, but the potential for uncontrolled growth – and net harm – remains. If Medicare covers lung cancer screening, it must require routine reporting of recruitment, screening, diagnosis, and treatment of lung cancer from every screening site. The findings will help patients decide about screening, set benchmarks for evaluating programs, and inform policy about screening.” The Veterans Health Administration is also implementing screening with data collection which will help “assess whether the predominantly older, male VHA population with several chronic conditions has different outcomes with screening from those of the younger, healthier NLST population” (Kinsinger, 2014).
With concerns and limitations, we find that the evidence is sufficient to conclude that lung cancer screening is reasonable and necessary for prevention or early detection of illness or disability, for Medicare beneficiaries under constraints similar to the NLST protocol, with specific eligibility requirements, performed by radiologists with specific LDCT training, and performed at qualified centers where specific data are collected. We note that LDCT screening has never been tested broadly in community settings or implemented on a widespread scale. Consistent with the USPSTF grade B recommendation, this programmatic approach and implementation framework allows LDCT lung cancer screening to be reasonable and necessary for Medicare beneficiaries. This approach was supported by evidence-based guidelines developed by multi-society multi-disciplinary stakeholders (Mazzone, 2014), and the two requestors of this national coverage determination. During the second comment period we received 133 comments, of which a large majority supported the framework of our proposed national coverage determination. Specific coverage criteria and other requirements have been adjusted accordingly in this final national coverage determination based on the evidence reviewed, including clarifications of published data and professional medical society position statements.
Question 3: Is the evidence sufficient to determine that screening for lung cancer with low dose computed tomography is appropriate for Medicare beneficiaries?
Lung cancer is a burdensome condition for the Medicare population due to the advanced stage and age at diagnosis and at death. Early detection with various screening tests has been studied for many years without positive results until the NLST. Based on the results of the NLST, lung cancer screening using LDCT was shown to reduce lung cancer mortality compared to screening with chest radiogram (x-ray) for a very defined screening population including a portion of the Medicare population. Since no other lung cancer screening test has been proven, there is no gold standard comparator to calculate sensitivity or specificity. Autopsy studies have not been published but may provide additional information in the future. The LDCT scan is relatively simple and attempts to balance radiation exposure to acceptable image quality, compared to standard diagnostic chest CT scans that are not appropriate for screening.
Medicare Beneficiary Eligibility
As noted above, the positive evidence for lung cancer screening with LDCT is from the NLST, with inclusion criteria limited to individuals aged 55 to 74 years who have &#≥ 30 pack-year smoking history and currently smoke or have quit smoking within the past 15 years. Many commenters asked for a reassessment of the age range for screening. Our proposed age range was set to match the enrollment age criterion of NLST. As these participants continued with the three annual screenings, however, benefit and harms data were actually recorded on participants up to and including the age of 77 years. In age specific analysis (Pinsky, 2014), no statistically significant increase in harms was reported. Data on these patients were analyzed along with the other trial participants to demonstrate overall benefits. Actual data from a well conducted randomized controlled trial provides the strongest form of evidence. From the NLST, CMS finds sufficient evidence to support an expansion of appropriate LDCT screening for eligible beneficiaries 55 to 77 years of age. There are no data and no evidence to show improvements in health outcomes or that benefits
outweigh harms, in smokers 78 years and older. Uncertain results from modeling based on unrealistic assumptions are insufficient.
Based on the evidence from the NLST, lung cancer screening with LDCT is only appropriate for Medicare beneficiaries as defined by the NLST and provided in a screening program.
Smoking History and Pack-Year Calculation
Smoking history is an important criterion for lung cancer screening using LDCT but is subject to recall bias (Bernaards, 2001; Krall, 1989; Mant, 2000; Persson, 1989; Prignot, 1987). Providers must ascertain smoking history accurately, taking into consideration intermittent periods of not smoking or reduced amounts, to ensure optimal screening outcomes.
One pack-year is equal to one pack of 20 cigarettes smoked each and every day for one year (Prignot, 1987).
Counseling and Shared Decision Making
Lung cancer screening with LDCT is a complex topic as noted above. Additionally, as discussed by Mazzone and colleages (2014) regarding who is offered lung cancer screening: “The principal question is how do lung cancer screening programs identify a group at high enough risk of developing lung cancer to benefit more than they are harmed. The balance with this choice is that more lives can be saved by screening at lower thresholds of risk, but the relative harms of screening increase as the threshold is lowered. It is difficult to determine the ideal balance of benefit and harm as the value of the benefit and harms is not equal, and varies with patient preferences.” Due to the complex factors involved in deciding whether to start a lung cancer screening program, formal shared decision making between the physician or non-physician practitioner and beneficiary is important. Eligible beneficiaries should be aware of, among other things, the individual commitment needed to gain benefits from a lung cancer screening program.
Barry and Edgman-Levitan (2012) noted: “[t]he process by which the optimal decision may be reached for a patient at a fateful health crossroads is called shared decision making and involves, at minimum, a clinician and the patient, although other members of the health care team or friends and family members may be invited to participate. In shared decision making, both parties share information: the clinician offers options and describes their risks and benefits, and the patient expresses his or her preferences and values. Each participant is thus armed with a better understanding of the relevant factors and shares responsibility in the decision about how to proceed. (Charles, 1997)”
Due to the specific patient selection criteria, benefits, harms, and adherence, along with the need for shared decision making, we believe that the lung cancer LDCT screening benefit should include a specific counseling and shared decision making visit that addresses the following:
- Determination of beneficiary eligibility including age, absence of signs or symptoms of lung cancer, a specific calculation of cigarette smoking pack-years; if former smoker, the number of years since quitting;
- Shared decision making, including the use of one or more decision aids, to include the benefits and harms of screening, including follow-up diagnostic testing, over-diagnosis, false positive rate, and total radiation exposure;
- Counseling on the importance of adherence to annual lung cancer LDCT screening, impact of comorbidities and ability or willingness to undergo diagnosis and treatment;
- Counseling on the importance of maintaining cigarette smoking abstinence if former smoker, or beginning/continuing tobacco use cessation if current smoker, and furnishing of information about tobacco cessation interventions.
- If appropriate, the furnishing of a written order for lung cancer screening with LDCT that includes: the beneficiary’s date of birth; current smoking status, actual pack-year smoking history (number); if former smoker, the number of years since quitting smoking; a statement that the beneficiary is asymptomatic (no signs or symptoms of lung cancer); and NPI of the ordering practitioner.
Decision aids may assist in this process. In a Cochrane Review, Stacey et al., noted “[a]ccording to the International Patient Decision Aids Standards (IPDAS) Collaboration, decision aids are evidence-based tools designed to help patients to participate in making specific and deliberated choices among healthcare options. Patient decision aids supplement (rather than replace) clinicians’ counselling about options. The specific aims of decision aids and the
type of decision support they provide may vary slightly, but in general they:
- Explicitly state the decision that needs to be considered;
- Provide evidence-based information about a health condition, the options, associated benefits, harms, probabilities, and scientific uncertainties;
- Help patients to recognize the values-sensitive nature of the decision and to clarify, either implicitly or explicitly, the value they place on the benefits, harms, and scientific uncertainties. (To accomplish this, patient decision aids may describe the options in enough detail that clients can imagine what it is like to experience the physical, emotional, and social effects and/or guide clients to consider which benefits and harms are most important to them).”
As part of the counseling and shared decision making visit, we are requiring that, among other things, shared decision making (including the use of one or more decision aids) includes information on benefits, harms, follow-up diagnostic testing, over-diagnosis, false positive rate and total radiation exposure. We believe that specific decision aids for lung cancer screening are being developed and will become more readily available as screening is more broadly adopted. While we are not requiring the use of a specific decision aid under this NCD, we note that the National Institutes of Health’s National Cancer Institute has developed a decision aid/tool, which is available at: http://www.cancer.gov/newscenter/qa/2002/NLSTstudyGuidePatientsPhysicians.
Reading Radiologist Training
Specific training and experience in performing LDCT screening and in interpreting results are important. The NLST had specific requirements for participating radiologists. Donnelly noted: “[l]ung cancer screening computed tomographies (CTs) differ from traditional chest CT scans in that they are performed at very low radiation doses, which allow the detection of small nodules but which have a much higher noise content that would not be acceptable in a diagnostic chest CT. The technical parameters require a great deal of attention on the part of the user, because inappropriate settings could result in either excess radiation dose to the large population of screened individuals or in low-quality images with impaired nodule detectability. Both situations undermine the main goal of the screening program, which is to detect lung nodules using as low a radiation dose as can reasonably be achieved. Once an image has been obtained, there are unique interpretive issues that must be addressed mainly because of the very high noise content of the images and the high prevalence of incidental findings in the chest unrelated to the sought-after pulmonary nodules.”
To ensure that benefits outweigh the harms, specific furnishing and interpreting radiologist training is needed, consistent with the NLST. “Approval for NLST also required review of a dedicated NLST training set, created by a subgroup of radiologists, which described acceptable CT and chest radiographic acquisition parameters and image quality requirements, and provided examples of various focal lung opacities with corresponding interpretations to promote a uniform knowledge base across sites.” (NLST, Radiology 2011) “Radiologist qualifications required certification by the American Board of Radiology or equivalent, documented training in diagnostic radiology and radiation safety, involvement in the supervision and interpretation of at least 300 chest CT acquisitions in the past 3 years and at least 200 chest radiographic acquisitions per year, and participation in continuing medical education in accordance with the American College of Radiology standard. Approval for NLST also required review of a dedicated NLST training set, created by a subgroup of radiologists, which described acceptable CT and chest radiographic acquisition parameters and image quality requirements, and provided examples of various focal lung opacities with corresponding interpretations to promote a uniform knowledge base across sites.” (NLST, Radiology 2011. Supplemental Materials)
The American College of Radiology requires for CT accreditation board certification in radiology or diagnostic radiology or for radiologists that are not board certified “interpretation and reporting of 500 CT examinations in the past 36 months’ and other medical education (http://www.acr.org/~/media/ACR/Documents/Accreditation/CT/Requirements.pdf). To be an ACR Designated Lung Cancer Screening Center, the radiologist must have experience of interpreting “200 chest CT cases in prior 36 months:” (http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Lung%20Screening/Lung%20Cancer%20Screening%20Center%20Attestation%20Form.pdf).
Radiation Dose
An important aspect of LDCT screening is reducing radiation exposure to as low as reasonably achievable (ALARA; see http://www.nrc.gov/reading-rm/basic-ref/glossary/alara.html), especially given the annual screening frequency and likely follow-up diagnostic imaging tests. Donnelly noted: [i]n general, lower radiation doses can be achieved by using lower tube currents and lower tube voltages. The current-time product (mAs) can be considered as the number of x-rays that enter the patient. Use of fewer x-rays (lower mAs) results in a lower radiation dose to the patient but in a higher noise content to the image. Typical tube current-time product values for diagnostic studies vary greatly depending upon the institution, patient size, and other scanning parameters; however, these values are often in the 200 to 250 mAs range for an average-sized patient. In the NLST study, screening lung cancer CT scans were typically performed at 20 to 40 mAs.”
“Although the acquisition parameters for low-dose CT are not explicitly defined in the imaging community, each of the scanners used in NLST was individually tested by using a CT dose index phantom and patient data to achieve comparable subjective image quality by using tube current–time products of 40 mAs or lower for the average-sized patient. Depending on the scanner, the effective tube current–time product (tube current–time products/scan pitch) range varied from 20 to 30 mAs for an average sized patient. It is estimated that each NLST low-dose CT resulted in an average effective dose of 1.5 mSv, whereas the effective dose from conventional chest CT varies considerably in clinical practice but is on
the order of 8 mSv.” (NLST, 2011 Radiology; Cody, 2010)
We received comments suggesting that the radiation dose should be as low as possible, and that CMS should allow for adjustments based on the size of the individual, along with suggesting that milligray (mGy) is a more common measurement than millisievert (mSv) for expressing volumetric dose. Based on the comments received and the evidence reviewed, including recently published multi-society, multi-disciplinary evidence-based recommendations (Kazerooni et al., 2014), we believe that LDCT with a volumetric CT dose index (CTDIvol) of ≤ 3.0 mGy (milligray) for standard size patients (defined to be 5’ 7” tall; and approximately 155 pounds) with appropriate reductions in CTDIvol for smaller patients and appropriate increases in CTDIvol for larger patients, is appropriate for this Medicare benefit.
LDCT Lung Cancer Screening Imaging Facilities
Over the past few months, multi-society multi-disciplinary stakeholders, including major professional medical societies and NLST investigators, have worked collaboratively and intently to develop evidence-based recommendations on the necessary components of a high quality lung cancer screening program. Two recent published articles present guidance from these major stakeholders. Kazerooni et al. (2014) published the recommendations of the American College of Radiology with support in public comments from the Lung Cancer Alliance and the Society of Thoracic Surgeons. Mazzone et al. (2014) published recommendations from multispecialty stakeholders including the American College of Chest Physicians, American Thoracic Society, American Association of Thoracic Surgery, American Cancer Society and the American Society of Preventive Oncology. These evidence-based recommendations describe components that are necessary for high quality LDCT lung cancer screening programs. As reported, these components are:
Component 1: Who is offered LDCT lung cancer screening;
Component 2: How often, and for how long, to screen;
Component 3: How the CT is performed;
Component 4: Lung nodule identification;
Component 5: Structured reporting;
Component 6: Lung nodule management algorithms;
Component 7: Smoking cessation;
Component 8: Patient and provider education;
Component 9: Data collection.
Based on these recently published evidence-based recommendations, CMS strongly encourages eligible facilities to implement the necessary components of a high quality LDCT lung cancer screening program as recommended by multi-society stakeholders. In addition, we support the development of a multi-society, multi-disciplinary governance body to continue to refine and optimize screening practices over time. CMS would gladly participate.
We received feedback from commenters regarding the proposed imaging facility eligibility criteria. While we agree with some of the commenters that requiring past participation in the NLST or advanced diagnostic imaging center accreditation with experience in LDCT lung cancer screening would place an undue burden on imaging facilities, we also agree with the commenters who expressed concern that without quality requirements, beneficiaries could be exposed to additional harms. Based on the evidence reviewed, in response to the public comments, and to ensure appropriate access to these services, we have modified the imaging facility eligibility criteria. We believe these revised imaging facility eligibility requirements strike an appropriate balance between maintaining appropriate and high quality access to these services, while reducing burden.
For purposes of Medicare coverage of lung cancer screening with LDCT, an eligible imaging facility is one that:
- Performs LDCT with volumetric CT dose index (CTDIvol) of ≤ 3.0 mGy (milligray) for standard size patients (defined to be 5’ 7” and approximately 155 pounds) with appropriate reductions in CTDIvol for smaller patients and appropriate increases in CTDIvol for larger patients;
- Utilizes a standardized lung nodule identification, classification, and reporting system;
- Makes available smoking cessation interventions for current smokers; and
- Collects and submits data as described in this NCD to a CMS-approved national registry for each LDCT lung cancer screening performed.
LDCT Lung Cancer Screening Registry
The primary purpose for requiring the submission of data to the registry is to document compliance with the coverage criteria that are not evidenced on the health care claim. Furthermore, based on the public comments and the evidence reviewed, we strongly believe that the registry will serve as an aid to those seeking to study the clinical benefits of this screening. The registry and the other criteria required in this NCD are supported by the evidence reviewed, including the NLST. The registry will help ensure that only eligible beneficiaries will receive this screening service since only beneficiaries that meet the eligibility requirements will benefit from such screening.
Furthermore, we recognize the impact of this criterion for imaging facilities. We will only require production of the minimum number of data elements to carry out this payment function in an effort to reduce burdens. Therefore, we are modifying the data registry elements, based on our review of the evidence and feedback received from commenters. As amended, the data elements are limited to those required to determine whether an individual has met the coverage criteria for the LDCT lung cancer screening service, that is, whether their receipt of the service was “reasonable and necessary” and “appropriate.” Data collected and submitted to a CMS-approved national registry must include, at minimum, all of the following elements:
| Data Type |
Minimum Required Data Elements |
| Facility |
Identifier |
| Radiologist (reading) |
National Provider Identifier (NPI) |
| Patient |
Identifier |
| Ordering Practitioner |
National Provider Identifier (NPI) |
| CT scanner |
Manufacturer, Model |
| Indication |
Lung cancer LDCT screening – absence of signs or symptoms of lung cancer |
| System |
Lung nodule identification, classification and reporting system |
| Smoking history |
Current status (current, former, never),
If former smoker, years since quitting,
Pack-years as reported by the ordering practitioner,
For current smokers, smoking cessation interventions available. |
| Effective radiation dose |
CT Dose Index (CTDIvol) |
| Screening |
Screen date
Initial Screen or
Subsequent Screen |
Additionally, national registries are strongly encouraged to collect data on lung nodules (for example: clinically significant non-lung cancer findings, the number and types of nodules, and size and location of each nodule), subsequent diagnostic testing, adverse events, and intermediate and long term health outcomes, in order to inform practices and policymakers about the ability to implement a LDCT lung cancer screening program broadly in multiple settings across the country, and achieve positive outcomes, consistent with the NLST. We recognize that these other data elements are extremely important to establishing the benefit of these screening services and improvement in health outcomes. We strongly encourage submission of such data elements to registries in addition to the minimum elements required under this NCD. These data will not only verify that screening leads to improved health outcomes for the Medicare population, but will also serve as the basis to refine and improve screening in practice, and serve the quality improvement purposes of screening facilities. We believe that multi-society stakeholders are in the best position to determine the appropriate data elements for reaching these goals, and to adjust the particular elements over time.
All CMS-approved registries must have the capacity and capability to collect data from any Medicare-eligible imaging facility/department that furnishes lung cancer screening with LDCT, with a catchment area that includes all 50 States, United States Territories, and the District of Columbia. CMS will evaluate each entity interested in participating as a CMS-approved registry to determine if they are capable of meeting the registry and data collection requirements outlined in this national coverage determination, including:
- Establishment of a steering committee and a governance board for oversight of the registry;
- Registry management plan, including identification of key personnel;
- Operational plan and framework that describes mechanisms for collection and submission of data from imaging facilities to the registry;
- Registry catchment area;
- Mechanisms for the submission of registry data to CMS electronically;
- Mechanisms to collect information (e.g.; HICN) in order to permit linkage of registry data with external databases (e.g. Medicare claims data sets);
- Description of data management and data quality review methods, including validation;
- Use of CMS-approved standardized data dictionary;
- Mechanisms for submitting a list of facilities participating in the registry to CMS; and
- Quality assurance plan.
Information regarding CMS-approved registries will be posted on the CMS website.
Adherence to Screening, Interval and Cumulative Screens
The NLST evaluated three annual LDCT screenings. DANTE and DLSCT evaluated five annual screenings. There is some consistency in the annual interval of screening studied in trials although there is little evidence to support an annual screen versus every two years or other interval. Results of ongoing trials in Europe may provide additional data in the future (Field, 2013). While the total number of annual screenings in these trials was likely influenced by trial logistics and funding, in addition to clinical considerations, the frequency and duration are important factors in adherence to a screening program over time. Adherence to screening protocols and programs is important to continually detect nodules over the study period. Adherence in the NLST was very high for all three screenings - “95 % in the low-dose CT group and 93 % in the radiography group” (NLST, 2011). If adherence is lower in general practice outside the clinical trial settings, the benefits of lung cancer screening, as demonstrated in the NLST, may be diminished.
Smoking Cessation
Since “[s]moking is widely recognized as the leading cause of lung cancer” (NCI - http://seer.cancer.gov/statfacts/html/lungb.html), smoking cessation interventions were integral interventions in published trials. According to the 2011 Medicare Current Beneficiary Survey (MCBS), 14 percent of beneficiaries reported to be current smokers, and 44 percent reported as former smokers, although pack-year information is not collected. Additionally, we received public comments that also addressed tobacco cessation interventions. Therefore, based on the evidence reviewed and public comments received, we are modifying the imaging facility eligibility criteria to require that smoking cessation interventions, such as educational materials, be made available.
Additional Considerations
Expenditure Assessment
As noted in 42 CFR § 410.64, an assessment of the relation between predicted outcome and the expenditures of an additional preventive service may be conducted and the results of such assessment may be taken into consideration in making a national coverage determination regarding the coverage of a new preventive service. Cost and cost-effectiveness analyses have been conducted and published. McMahon et al. (2011) reported that “[a]nnual screening of current and former smokers aged 50–74 cost between $126,000–$169,000/QALY (minimum 20 pack-years of smoking) or $110,000–$166,000/QALY (40 pack-year minimum), compared to no screening and assuming background quit rates” and concluded that “[t]he cost-effectiveness of CT screening will likely be strongly linked to achievable smoking cessation rates. Trials and further modeling should explore the consequences of relationships between smoking behaviors and screen participation.” Black et al. (2014) estimated that incremental cost-effectiveness ratios (ICERs) were $52,000 per life-year gained (95 % CI, 34,000 to 106,000). CMS will continue to closely monitor publications on expenditure assessments of lung cancer screening with LDCT.
Post Coverage Analysis
In addition to the data to be collected to confirm screening eligibility, provider and center requirements, CMS will monitor benefits and harms using administrative and clinical databases such as SEER and/or U.S. Cancer Statistics as necessary to ensure that benefits continue to outweigh harms when lung cancer LDCT screening is implemented broadly outside the controlled trial setting, and when screening data accumulates beyond the three annual screens seen in the NLST. Without continuing outcomes evaluation, there is insufficient evidence to support screening greater than three annual LDCT lung cancer screenings. CMS will evaluate five year data to better establish duration and the cumulative limit in the number of LDCT screens, as well as inform, if appropriate, modifications to Medicare coverage.
Disparities
Lung cancer incidences are more common in men than women, and highest in African American men. Women comprised 41 percent of the study population in NLST. We note that 90.9 percent of participants in the NLST were white. CMS encourages shared decision making between patients and practitioners, and if appropriate, participation in a lung cancer screening program.
Number of New Cases per 100,000 Persons by Race/Ethnicity & Sex: Lung and Bronchus Cancer (SEER 18, 2007-2011, Age-Adjusted)
http://seer.cancer.gov/statfacts/html/lungb.html
Summary
Given the burden of lung cancer on the United States population, a suitable screening test for lung cancer has been sought for many years. Lung cancer screening has been recommended by the USPSTF with a grade B recommendation for certain individuals. Based on our review of the available evidence, including clinical guidelines and public comments, we find that the evidence is sufficient to conclude that lung cancer screening with LDCT is reasonable and necessary for prevention or early detection of illness or disability and appropriate for Medicare beneficiaries under conditions established in this NCD. These conditions are supported by the evidence reviewed, including conditions in the NLST and evidence-based multi-society, multi-disciplinary recommendations. The results of ongoing trials will provide additional evidence. We believe that specific beneficiary, practitioner, and imaging facility eligibility requirements are necessary to ensure that benefits of screening outweigh harms in the Medicare population, consistent with the NLST. Lung cancer screening with LDCT has not been implemented broadly in any population to date. While we are establishing coverage for this additional preventive service under Medicare part B, we believe we need to proceed in a responsible manner and will continue to monitor the evidence as stewards of the Medicare program.
IX. Conclusion
The Centers for Medicare & Medicaid Services (CMS) has determined that the evidence is sufficient to add a lung cancer screening counseling and shared decision making visit, and for appropriate beneficiaries, annual screening for lung cancer with low dose computed tomography (LDCT), as an additional preventive service benefit under the Medicare program only if all of the following criteria are met:
Beneficiary eligibility criteria:
- Age 55 – 77 years;
- Asymptomatic (no signs or symptoms of lung cancer);
- Tobacco smoking history of at least 30 pack-years (one pack-year = smoking one pack per day for one year; 1 pack = 20 cigarettes);
- Current smoker or one who has quit smoking within the last 15 years; and
- Receives a written order for LDCT lung cancer screening that meets the following criteria:
-
For the initial LDCT lung cancer screening service: a beneficiary must receive a written order for LDCT lung cancer screening during a lung cancer screening counseling and shared decision making visit, furnished by a physician (as defined in Section 1861(r)(1) of the Social Security Act) or qualified non-physician practitioner (meaning a physician assistant, nurse practitioner, or clinical nurse specialist as defined in §1861(aa)(5) of the Social Security Act). A lung cancer screening counseling and shared decision making visit includes the following elements (and is appropriately documented in the beneficiary’s medical records):
- Determination of beneficiary eligibility including age, absence of signs or symptoms of lung cancer, a specific calculation of cigarette smoking pack-years; and if a former smoker, the number of years since quitting;
- Shared decision making, including the use of one or more decision aids, to include benefits and harms of screening, follow-up diagnostic testing, over-diagnosis, false positive rate, and total radiation exposure;
- Counseling on the importance of adherence to annual lung cancer LDCT screening, impact of comorbidities and ability or willingness to undergo diagnosis and treatment;
- Counseling on the importance of maintaining cigarette smoking abstinence if former smoker; or the importance of smoking cessation if current smoker and, if appropriate, furnishing of information about tobacco cessation interventions; and
- If appropriate, the furnishing of a written order for lung cancer screening with LDCT.
- For subsequent LDCT lung cancer screenings: the beneficiary must receive a written order for LDCT lung cancer screening, which may be furnished during any appropriate visit with a physician (as defined in Section 1861(r)(1) of the Social Security Act) or qualified non-physician practitioner (meaning a physician assistant, nurse practitioner, or clinical nurse specialist as defined in Section 1861(aa)(5) of the Social Security Act). If a physician or qualified non-physician practitioner elects to provide a lung cancer screening counseling and shared decision making visit for subsequent lung cancer screenings with LDCT, the visit must meet the criteria described above for a counseling and shared decision making visit.
-
Written orders for both initial and subsequent LDCT lung cancer screenings must contain the following information, which must also be appropriately documented in the beneficiary’s medical records:
- Beneficiary date of birth;
- Actual pack - year smoking history (number);
- Current smoking status, and for former smokers, the number of years since quitting smoking;
- Statement that the beneficiary is asymptomatic (no signs or symptoms of lung cancer); and
- National Provider Identifier (NPI) of the ordering practitioner.
Reading radiologist eligibility criteria:
- Board certification or board eligibility with the American Board of Radiology or equivalent organization;
- Documented training in diagnostic radiology and radiation safety;
- Involvement in the supervision and interpretation of at least 300 chest computed tomography acquisitions in the past 3 years;
- Documented participation in continuing medical education in accordance with current American College of Radiology standards; and
- Furnish lung cancer screening with LDCT in a radiology imaging facility that meets the radiology imaging facility eligibility criteria below.
Radiology imaging facility eligibility criteria:
- Performs LDCT with volumetric CT dose index (CTDIvol) of ≤ 3.0 mGy (milligray) for standard size patients (defined to be 5’ 7” and approximately 155 pounds) with appropriate reductions in CTDIvol for smaller patients and appropriate increases in CTDIvol for larger patients;
- Utilizes a standardized lung nodule identification, classification and reporting system;
- Makes available smoking cessation interventions for current smokers; and
- Collects and submits data to a CMS-approved registry for each LDCT lung cancer screening performed. The data collected and submitted to a CMS-approved registry must include, at minimum, all of the following elements:
| Data Type |
Minimum Required Data Elements |
| Facility |
Identifier |
| Radiologist (reading) |
National Provider Identifier (NPI) |
| Patient |
Identifier |
| Ordering Practitioner |
National Provider Identifier (NPI) |
| CT scanner |
Manufacturer, Model. |
| Indication |
Lung cancer LDCT screening – absence of signs or symptoms of lung cancer
|
| System |
Lung nodule identification, classification and reporting system |
| Smoking history |
Current status (current, former, never).
If former smoker, years since quitting.
Pack-years as reported by the ordering practitioner.
For current smokers, smoking cessation interventions available.
|
| Effective radiation dose |
CT Dose Index (CTDIvol).
|
| Screening |
Screen date
Initial screen or subsequent screen |
All CMS-approved registries must have the capacity and capability to collect data from any Medicare-eligible imaging facility/department that furnishes lung cancer screening with LDCT, with a catchment area that includes all 50 States, United States Territories, and the District of Columbia. CMS will evaluate each entity interested in participating as a CMS-approved registry to determine if they are capable of meeting the registry and data collection requirements outlined in this national coverage determination, including:
- Establishment of a steering committee and a governance board for oversight of the registry;
- Registry management plan, including identification of key personnel;
- Operational plan and framework that describes mechanisms for collection and submission of data from imaging facilities to the registry;
- Registry catchment area;
- Mechanisms for the submission of registry data to CMS electronically;
- Mechanisms to collect information (e.g.; HICN) in order to permit linkage of registry data with external databases (e.g. Medicare claims data sets);
- Description of data management and data quality review methods, including validation;
- Use of CMS-approved standardized data dictionary;
- Mechanisms for submitting a list of facilities participating in the registry to CMS; and
- Quality assurance plan.
To apply to function as a CMS-approved registry, interested entities must submit a letter of interest along with detailed supporting information about how the interested entity is able to meet the requirements outlined in this national coverage determination to the following address or via email to caginquiries@cms.hhs.gov.
Centers for Medicare & Medicaid Services
Center for Clinical Standards and Quality
Director, Coverage and Analysis Group
ATTN: Lung Cancer LDCT Screening
Mail Stop: S3-02-01
7500 Security Blvd.
Baltimore, MD 21244
Information regarding CMS-approved registries will be posted on the CMS website.
Appendix A
Articles/References Submitted by the Requestors
Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England journal of medicine 2011;365:395-409.
Aberle DR, Adams AM, Berg CD, et al. Baseline characteristics of participants in the randomized national lung screening trial. Journal of the National Cancer Institute 2010;102:1771-9.
Aberle DR, Berg CD, Black WC, et al. The National Lung Screening Trial: overview and study design. Radiology 2011;258:243-53.
Ashraf H, Tonnesen P, Holst Pedersen J, Dirksen A, Thorsen H, Dossing M. Effect of CT screening on smoking habits at 1-year follow-up in the Danish Lung Cancer Screening Trial (DLCST). Thorax 2009;64:388-92.
Bach PB. Perilous Potential: The Chance to Save Lives, or Lose them, Through Low Dose Computed Tomography Screening for Lung Cancer Journal of Surgical Oncology 2013;In press.
Bach PB, Gould MK. When the average applies to no one: personalized decision making about potential benefits of lung cancer screening. Annals of internal medicine 2012;157:571-3.
Bach PB, Kattan MW, Thornquist MD, et al. Variations in lung cancer risk among smokers. Journal of the National Cancer Institute 2003;95:470-8.
Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA : the journal of the American Medical Association 2012;307:2418-29.
Baldwin DR, Duffy SW, Wald NJ, Page R, Hansell DM, Field JK. UK Lung Screen (UKLS) nodule management protocol: modelling of a single screen randomised controlled trial of low-dose CT screening for lung cancer. Thorax
2011;66:308-13.
Bastarrika G, Garcia-Velloso MJ, Lozano MD, et al. Early lung cancer detection using spiral computed tomography and positron emission tomography. Am J Respir Crit Care Med 2005;171:1378-83.
Becker N, Motsch E, Gross ML, et al. Randomized study on early detection of lung cancer with MSCT in Germany: study design and results of the first screening round. Journal of cancer research and clinical oncology 2012;138:1475-86.
Blanchon T, Brechot JM, Grenier PA, et al. Baseline results of the Depiscan study: a French randomized pilot trial of lung cancer screening comparing low dose CT scan (LDCT) and chest X-ray (CXR). Lung Cancer 2007;58:50-8.
Callol L, Roig F, Cuevas A, et al. Low-dose CT: a useful and accessible tool for the early diagnosis of lung cancer in selected populations. Lung Cancer 2007;56:217-21.
Cassidy A, Myles JP, van Tongeren M, et al. The LLP risk model: an individual risk prediction model for lung cancer. British journal of cancer 2008;98:270-6.
de Koning HJ, Meza R, Plevritis SK, et al. Benefits and Harms of Computed Tomography Lung Cancer Screening Programs for High-Risk Populations. 2013.
Diederich S, Thomas M, Semik M, et al. Screening for early lung cancer with low-dose spiral computed tomography: results of annual follow-up examinations in asymptomatic smokers. Eur Radiol 2004;14:691-702.
Diederich S, Wormanns D, Lenzen H, Semik M, Thomas M, Peters PE. Screening for asymptomatic early bronchogenic carcinoma with low dose CT of the chest. Cancer 2000;89:2483-4.
Diederich S, Wormanns D, Semik M, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology 2002;222:773-81.
Garg K, Keith RL, Byers T, et al. Randomized controlled trial with low-dose spiral CT for lung cancer screening: feasibility study and preliminary results. Radiology 2002;225:506-10.
Gohagan J, Marcus P, Fagerstrom R, Pinsky P, Kramer B, Prorok P. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: the Lung Screening Study of the National Cancer Institute. Chest 2004;126:114-21.
Gohagan JK, Marcus PM, Fagerstrom RM, et al. Final results of the Lung Screening Study, a randomized feasibility study of spiral CT versus chest X-ray screening for lung cancer. Lung Cancer 2005;47:9-15.
Henschke CI, Naidich DP, Yankelevitz DF, et al. Early lung cancer action project: initial findings on repeat screenings. Cancer 2001;92:153-9.
Henschke CI, Yankelevitz DF, Libby DM, et al. Early lung cancer action project: annual screening using single-slice helical CT. Ann N Y Acad Sci 2001;952:124-34.
Humphrey LL, Deffebach M, Pappas M, et al. Screening for Lung Cancer With Low-Dose Computed Tomography: A Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Annals of internal medicine 2013.
Infante M, Cavuto S, Lutman FR, et al. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med 2009;180:445-53.
Infante M, Chiesa G, Solomon D, et al. Surgical procedures in the DANTE trial, a randomized study of lung cancer early detection with spiral computed tomography: comparative analysis in the screening and control arm. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2011;6:327-35.
Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. The Journal of thoracic and cardiovascular surgery 2012;144:33-8.
Lopes Pegna A, Picozzi G, Mascalchi M, et al. Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low-dose CT. Lung Cancer 2009;64:34-40.
Lung Cancer Alliance. LCA and Others Support High Grade for Screening. Available online at: http://www.lungcanceralliance.org/news/legislative-activities/2013/lca-and-others-support-high-grade-for-screening.html.
Lung Cancer Alliance. National Framework for Excellence in Lung Cancer Screening and Continuum of Care. Available online at: http://www.lungcanceralliance.org/am-i-at-risk/national-framework-for-lung-screening-excellence.html.
Lung Cancer Screening Draft Recommendation Statement. AHRQ Publication No. 13-05196-EF-3. 2013. (Accessed 9/6/2013, at http://www.uspreventiveservicestaskforce.org/uspstf13/lungcan/lungcandraftrec.htm.)
MacRedmond R, Logan PM, Lee M, Kenny D, Foley C, Costello RW. Screening for lung cancer using low dose CT scanning. Thorax 2004;59:237-41.
MacRedmond R, McVey G, Lee M, et al. Screening for lung cancer using low dose CT scanning: results of 2 year follow up. Thorax 2006;61:54-6.
Menezes RJ, Roberts HC, Paul NS, et al. Lung cancer screening using low-dose computed tomography in at-risk individuals: the Toronto experience. Lung Cancer 2010;67:177-83.
NCCN Clinical Practice Guidelines in Oncology: Lung Cancer Screening (Version 1.2014). (Accessed 8/14/2013, at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#lung_screening.)
Novello S, Fava C, Borasio P, et al. Three-year findings of an early lung cancer detection feasibility study with low-dose spiral computed tomography in heavy smokers. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2005;16:1662-6.
Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA : the journal of the American Medical Association 2011;306:1865-73.
Pastorino U, Bellomi M, Landoni C, et al. Early lung-cancer detection with spiral CT and positron emission tomography in heavy smokers: 2-year results. Lancet 2003;362:593-7.
Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP) 2012;21:308-15.
Pedersen JH, Ashraf H, Dirksen A, et al. The Danish randomized lung cancer CT screening trial overall design and results of the prevalence round. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2009;4:608-14.
Picozzi G, Paci E, Lopez Pegna A, et al. Screening of lung cancer with low dose spiral CT: results of a three year pilot study and design of the randomised controlled trial ''Italung-CT''. Radiol Med 2005;109:17-26.
Providing Guidance on Lung Cancer Screening to Patients and Physicians. 2012. (Accessed 1/25/2013, at http://www.lung.org/lung-disease/lung-cancer/lung-cancer-screening-guidelines/lung-cancer-screening.pdf.)
Pyenson B, Sander M, Jiang Y, Kahn H, and Mulshine JL. An actuarial analysis shows that offering lung cancer screening as an insurance benefit would save lives at relatively low cost. Health Affairs. doi: 10.1377/hlthaff.2011.0814. No. 4 (2014):770-779.
Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax 2012;67:296-301.
Sobue T, Moriyama N, Kaneko M, et al. Screening for lung cancer with low-dose helical computed tomography: anti-lung cancer association project. J Clin Oncol 2002;20:911-20.
Spitz MR, Hong WK, Amos CI, et al. A risk model for prediction of lung cancer. Journal of the National Cancer Institute 2007;99:715-26.
Swensen SJ, Jett JR, Hartman TE, et al. CT screening for lung cancer: five-year prospective experience. Radiology 2005;235:259-65.
Swensen SJ, Jett JR, Hartman TE, et al. Lung cancer screening with CT: Mayo Clinic experience. Radiology 2003;226:756-61.
Swensen SJ, Jett JR, Sloan JA, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med 2002;165:508-13.
Tammemagi CM, Pinsky PF, Caporaso NE, et al. Lung cancer risk prediction: Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial models and validation. Journal of the National Cancer Institute 2011;103:1058-68.
Tammemagi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. The New England journal of medicine 2013;368:728-36.
van der Aalst CM, van den Bergh KA, Willemsen MC, de Koning HJ, van Klaveren RJ. Lung cancer screening and smoking abstinence: 2 year follow-up data from the Dutch-Belgian randomised controlled lung cancer screening trial. Thorax 2010;65:600-5.
van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. The New England journal of medicine 2009;361:2221-9.
Veronesi G, Bellomi M, Mulshine JL, et al. Lung cancer screening with low-dose computed tomography: a non-invasive diagnostic protocol for baseline lung nodules. Lung Cancer 2008;61:340-9.
Veronesi G, Bellomi M, Scanagatta P, et al. Difficulties encountered managing nodules detected during a computed tomography lung cancer screening program. The Journal of thoracic and cardiovascular surgery 2008;136:611-7.
Wender R, Fontham ET, Barrera E, Jr., et al. American Cancer Society lung cancer screening guidelines. CA: a cancer journal for clinicians 2013.
Wilson DO, Weissfeld JL, Fuhrman CR, et al. The Pittsburgh Lung Screening Study (PLuSS): outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med 2008;178:956-61.
Appendix B
References Submitted by Commenters
AAPM Lung Cancer Screening CT Protocols Version 2.0 Sept. 9 2014 http://www.aapm.org/pubs/CTProtocols/documents/LungCancerScreeningCT.pdf.
AAPM Report 204: Size-Specific Dose Estimates (SSDE) in Pediatric and Adult Body CT Examinations”,http://www.aapm.org/pubs/reports/RPT_204.pdf.
AAPM Report 220: Use of Water Equivalent Diameter for Calculating Patient Size and Size-Specific Dose Estimates (SSDE) in CT: Report of AAPM Task Group 220”, http://www.aapm.org/pubs/reports/RPT_220.pdf.
Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England journal of medicine. 2011;365(5):395-409.
ACR Designated Lung Cancer Screening Center Attestation Form, http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Lung%20Screening/Lung%20Cancer%20Screening%20Center%20Attestation%20Form.pdf
ACR Lung Cancer Screening Center, http://www.acr.org/Quality-Safety/Lung-Cancer-Screening-Center.
Alberg AJ, Brock M V, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(5 Suppl):e1S-29S. doi:10.1378/chest.12-2345.
Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, Byers T, Colditz GA, Gould MK, Jett JR, Sabichi AL, Smith-Bindman R, Wood DE, Qaseem A, Detterbeck FC. Benefits and harms of CT screening for lung cancer: a systematic review. Jama 2012; 307: 2418-2429.
Black WC, Gareen IF, Soneji SS, et al. Cost-Effectiveness of CT Screening in the National Lung Screening Trial. N. Engl. J. Med. 2014;371(19):1793-1802. doi:10.1056/NEJMoa1312547.
Brenner DR, McLaughlin JR, Hung RJ. Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PLoS One 2011;6(3):e17479. doi:10.1371/journal.pone.0017479.
Cagnon CH, Cody DD, McNitt-Gray MF, Seibert JA, Judy PF, Aberle DR. Description and implementation of a quality control program in an imaging-based clinical trial. Acad Radiol. 2006 Nov; 13(11):1431-41.PMID: 17111584.
Centers for Disease Control and Prevention, Lung Cancer, available at http://www.cdc.gov/cancer/lung/.
de Koning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med 2014;160:311-20.
Denholm R, Schüz J, Straif K, et al. Is previous respiratory disease a risk factor for lung cancer? Am. J. Respir. Crit. Care Med. 2014;190(5):549-59. doi:10.1164/rccm.201402-0338OC.
De Torres JP, Marín JM, Casanova C, et al. Lung Cancer in patients with COPD: Incidence and predicting factors. Am. J. Respir. Crit. Care Med. 2011;184(8):913-9. doi:10.1164/rccm.201103-0430OC.
Detterbeck FC. Cancer, concepts, cohorts and complexity: avoiding oversimplification of overdiagnosis. Thorax 2012; 67: 842-845.
Halpern MT, Gillespie BW, Warner KE. Patterns of absolute risk of lung cancer mortality in former smokers. Journal of the National Cancer Institute 1993;85:457-64.
Houghton AM, Mouded M, Shapiro SD. Common origins of lung cancer and COPD. Nat. Med. 2008;14(10):1023-4. doi:10.1038/nm1008-1023.
International Commission on Radiological Protection, “The 2007 Recommendations of the International Commission on Radiological Protection,” ICRP Publication 103 International Commission on Radiological Protection, Essen, 2007.
Jaklitsch MT, MD, Jacobson FL, MD, MPH, Austin JHM, MD et al. American Association for Thoracic Surgery (AATS) Guidelines for Lung Cancer Screening Using Low-dose Computed Tomography Scans for Lung Cancer Survivors and Other High-risk Groups. Journal of Thoracic and Cardiovascular Surgery 2012; July, Vol 144, No. 1 http://aats.org/multimedia/files/Guidelines/Lung-Cancer-Screening-Using-low-dose-computed-tomography-scans.pdf
Kahn JM, Gould MK, Krishnan JA, Wilson KC, Au DH, Cooke CR, Douglas IS, Feemster LC, Mularski RA, Slatore CG, Renda Soylemez W. An official american thoracic society workshop report: developing performance measures from clinical practice guidelines. Ann Am Thorac Soc 2014; 11: S186-195.
Kinsinger LS, Atkins D, Provenzale D, Anderson C, Petzel R. Implementation of a New Screening Recommendation in Health Care: The Veterans Health Administration's Approach to Lung Cancer ScreeningImplementation of a New Screening Recommendation in Health Care. Annals of Internal Medicine 2014; 161: 597-598.
Lillie SE, Partin MR, Rice K, Fabbrini AE, Greer NL, Patel S, MacDonald R, Rutks I, Wilt TJ. The Effects of Shared Decision Making on Cancer Screening - A Systematic Review. VA ESP Project #09-009 2014.
Mazzone P, Powell CA, Arenberg D, Bach P, Detterbeck F, Gould M, Jaklitsch MT, Jett J, Naidich D, Vachani A, Wiener RS, Silvestri G. Components Necessary for High Quality Lung Cancer Screening: American College of Chest Physicians and American Thoracic Society Policy Statement. Chest 2014.
McKee BJ, Hashim JA, French RJ, et al. Experience with a CT Screening Program for Individuals at High Risk for Developing Lung Cancer. Journal of the American College of Radiology : JACR. 2014
McKee, B. J., McKee, A. B., Flacke, S., Lamb, C. R., Hesketh, P. J., & Wald, C. (2013). Initial Experience With a Free, High-Volume, Low-Dose CT Lung Cancer Screening Program. Journal of the American College of Radiology.
Moyer et al. on behalf of the United States Preventive Services Task Force. Screening for Lung Cancer: U.S. Preventative Services Task Force Recommendation Statement. Annals of Internal Medicine 2014; doi: 4 March, Vol 160, No. 5 Moyer VA, MD, MPH on behalf of the U.S. Preventive Services Task Force http://annals.org/article.aspx?articleid=1809422&resultClick=3
National Cancer Institute: SEER State Fact Sheets: Lung and Bronchus Cancer, available at http://seer.cancer.gov/statfacts/html/lungb.html.
NCCN Clinical Practice Guidelines in Oncology (NCCN GuidelinesR): Lung Cancer Screening, v. 1.2015; http://www.nccn.org/professionalls/physician_gls/pdf/lung_screening.pdf.
NCCN Guidelines at MS-10
National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011 Aug 4;365(5):395-409. doi: 10.1056/NEJMoa1102873. Epub 2011 Jun 29. PMID: 21714641
Peto, J. (2011) That lung cancer incidence falls in ex-smokers: misconceptions 2. British Journal of Cancer, 104,389. doi:10.1038/sj.bjc.6606080.
Pinsky PF, Gierada DS, Hocking W, Patz EF, Kramer BS. National Lung Screening Trial Findings by Age: Medicare-Eligible Versus Under-65 Population. Ann. Intern. Med. 2014;161(9):627-633. doi:10.7326/M14-1484.
Pyenson BS, Sander MS, Jiang Y, et al. An Actuarial Analysis Shows That Offering Lung Cancer Screening as an Insurance Benefit Would Save Lives at Relatively Low Cost. Health Affairs 2012; 4:770–779.
Tammemägi MC, Church TR, Hocking WG, Silvestri GA, Kvale PA, et al. (2014) Evaluation of the Lung Cancer Risks at Which to Screen Ever- and Never-Smokers: Screening Rules Applied to the PLCO and NLST Cohorts. PLoS Med 11(12): e1001764. doi:10.1371/journal.pmed.1001764
US Department of Health and Human Services. The Health Consequences of Smoking - 50 Years of Progress: A Report of the Surgeon General. Atlanta, GA; 2014.
Vineis, P., Alavanja, M.,Buffler, P.,Fontham, E., Franceschi, S,Gao, Y. T.,Gupta, P. C.,Hackshaw, A., Matos, E.,Samet, J.,Sitas, F.,Smith, J.,Stayner, L.,Straif, K.,Thun, M. J.,Wichmann, H. E.,Wu, A. H., Zaridze, D.,Peto, R.,Doll, R. (2004). Tobacco and Cancer: Recent Epidemiological Evidence. Journal of the National Cancer Institute, 96(2), 99- 106. Doi:10.1093/jnci/djh014 http://jnci.oxfordjournals.org/content/96/2/99.short
Zulueta JJ, Wisnivesky JP, Henschke CI, et al. Emphysema Scores Predict Death from Chronic Obstructive Pulmonary Disease and Lung Cancer. Chest 2011. doi:10.1378/chest.11-0101.
This information is representative of Medicare's national coverage determination (NCD) for implementation purposes only. The information is subject to formal revisions and formatting changes prior to the release of the final NCD contractor instructions and publication in the Medicare National Coverage Determinations Manual.
Screening for Lung Cancer with Low Dose Computed Tomography (LDCT)
Item/Service Description
A. General
Lung cancer is the third most common cancer and the leading cause of cancer deaths in the United States. Cancer of the lung and bronchus accounted for over 150,000 deaths in 2013, with a median age at death of 72 years. Computed tomography (CT) is an imaging procedure that uses specialized x-ray equipment to create detailed pictures of areas inside the body. Low dose computed tomography (LDCT) is a chest CT scan performed at settings to minimize radiation exposure compared to a standard chest CT.
Under §1861(ddd) of the Social Security Act (the Act), the Centers for Medicare & Medicaid Services (CMS) has the authority to add coverage of additional preventive services if certain statutory requirements are met. The regulations provide:
42 CFR § 410.64 Additional preventive services
(a) Medicare Part B pays for additional preventive services not described in paragraph (1) or (3) of the definition of “preventive services” under §410.2, that identify medical conditions or risk factors for individuals if the Secretary determines through the national coverage determination process (as defined in section 1869(f)(1)(B) of the Act) that these services are all of the following: (1) reasonable and necessary for the prevention or early detection of illness or disability.(2) recommended with a grade of A or B by the United States Preventive Services Task Force, (3) appropriate for individuals entitled to benefits under Part A or enrolled under Part B.
(b) In making determinations under paragraph (a) of this section regarding the coverage of a new preventive service, the Secretary may conduct an assessment of the relation between predicted outcomes and the expenditures for such services and may take into account the results of such an assessment in making such national coverage determinations.
The scope of the national coverage analysis evaluated the evidence for screening for lung cancer with LDCT. Diagnostic CTs were outside the scope of the national coverage analysis.
Indications and Limitations of Coverage
B. Nationally Covered Indications
The Centers for Medicare & Medicaid Services (CMS) has determined that the evidence is sufficient to add a lung cancer screening counseling and shared decision making visit, and for appropriate beneficiaries, annual screening for lung cancer with low dose computed tomography (LDCT), as an additional preventive service benefit under the Medicare program only if all of the following criteria are met:
Beneficiary eligibility criteria:
Age 55 – 77 years;
Asymptomatic (no signs or symptoms of lung cancer);
Tobacco smoking history of at least 30 pack-years (one pack-year = smoking one pack per day for one year; 1 pack = 20 cigarettes);
Current smoker or one who has quit smoking within the last 15 years; and
Receives a written order for LDCT lung cancer screening that meets the following criteria:
-
For the initial LDCT lung cancer screening service: a beneficiary must receive a written order for LDCT lung cancer screening during a lung cancer screening counseling and shared decision making visit, furnished by a physician (as defined in Section 1861(r)(1) of the Social Security Act) or qualified non-physician practitioner (meaning a physician assistant, nurse practitioner, or clinical nurse specialist as defined in §1861(aa)(5) of the Social Security Act). A lung cancer screening counseling and shared decision making visit includes the following elements (and is appropriately documented in the beneficiary’s medical records):
- Determination of beneficiary eligibility including age, absence of signs or symptoms of lung cancer, a specific calculation of cigarette smoking pack-years; and if a former smoker, the number of years since quitting;
- Shared decision making, including the use of one or more decision aids, to include benefits and harms of screening, follow-up diagnostic testing, over-diagnosis, false positive rate, and total radiation exposure;
- Counseling on the importance of adherence to annual lung cancer LDCT screening, impact of comorbidities and ability or willingness to undergo diagnosis and treatment;
- Counseling on the importance of maintaining cigarette smoking abstinence if former smoker; or the importance of smoking cessation if current smoker and, if appropriate, furnishing of information about tobacco cessation interventions; and
- If appropriate, the furnishing of a written order for lung cancer screening with LDCT.
- For subsequent LDCT lung cancer screenings: the beneficiary must receive a written order for LDCT lung cancer screening, which may be furnished during any appropriate visit with a physician (as defined in Section 1861(r)(1) of the Social Security Act) or qualified non-physician practitioner (meaning a physician assistant, nurse practitioner, or clinical nurse specialist as defined in Section 1861(aa)(5) of the Social Security Act). If a physician or qualified non-physician practitioner elects to provide a lung cancer screening counseling and shared decision making visit for subsequent lung cancer screenings with LDCT, the visit must meet the criteria described above for a counseling and shared decision making visit.
- Written orders for both initial and subsequent LDCT lung cancer screenings must contain the following information, which must also be appropriately documented in the beneficiary’s medical records:
- Beneficiary date of birth;
- Actual pack - year smoking history (number);
- Current smoking status, and for former smokers, the number of years since quitting smoking;
- Statement that the beneficiary is asymptomatic (no signs or symptoms of lung cancer); and
- National Provider Identifier (NPI) of the ordering practitioner.
Reading radiologist eligibility criteria:
- Board certification or board eligibility with the American Board of Radiology or equivalent organization;
- Documented training in diagnostic radiology and radiation safety;
- Involvement in the supervision and interpretation of at least 300 chest computed tomography acquisitions in the past 3 years;
- Documented participation in continuing medical education in accordance with current American College of Radiology standards; and
- Furnish lung cancer screening with LDCT in a radiology imaging facility that meets the radiology imaging facility eligibility criteria below.
Radiology imaging facility eligibility criteria:
- Performs LDCT with volumetric CT dose index (CTDIvol) of ≤ 3.0 mGy (milligray) for standard size patients (defined to be 5’ 7” and approximately 155 pounds) with appropriate reductions in CTDIvol for smaller patients and appropriate
- increases in CTDIvol for larger patients;
- Utilizes a standardized lung nodule identification, classification and reporting system;
- Makes available smoking cessation interventions for current smokers; and
- Collects and submits data to a CMS-approved registry for each LDCT lung cancer screening performed. The data collected and submitted to a CMS-approved registry must include, at minimum, all of the following elements:
| Data Type |
Minimum Required Data Elements |
| Facility |
Identifier |
| Radiologist (reading) |
National Provider Identifier (NPI) |
| Patient |
Identifier |
| Ordering Practitioner |
National Provider Identifier (NPI) |
| CT scanner |
Manufacturer, Model. |
| Indication |
Lung cancer LDCT screening – absence of signs or symptoms of lung cancer |
| System |
Lung nodule identification, classification and reporting system |
|
Smoking history |
Current status (current, former, never).
If former smoker, years since quitting.
Pack-years as reported by the ordering practitioner.
For current smokers, smoking cessation interventions available. |
| Effective radiation dose |
CT Dose Index (CTDIvol).
|
| Screening |
Screen date
Initial screen or subsequent screen |
All CMS-approved registries must have the capacity and capability to collect data from any Medicare-eligible imaging facility/department that furnishes lung cancer screening with LDCT, with a catchment area that includes all 50 States, United States Territories, and the District of Columbia. CMS will evaluate each entity interested in participating as a CMS-approved registry to determine if they are capable of meeting the registry and data collection requirements outlined in this national coverage determination, including:
- Establishment of a steering committee and a governance board for oversight of the registry;
- Registry management plan, including identification of key personnel;
- Operational plan and framework that describes mechanisms for collection and submission of data from imaging facilities to the registry;
- Registry catchment area;
- Mechanisms for the submission of registry data to CMS electronically;
- Mechanisms to collect information (e.g.; HICN) in order to permit linkage of registry data with external databases (e.g. Medicare claims data sets);
- Description of data management and data quality review methods, including validation;
- Use of CMS-approved standardized data dictionary;
- Mechanisms for submitting a list of facilities participating in the registry to CMS; and
- Quality assurance plan.
To apply to function as a CMS-approved registry, interested entities must submit a letter of interest along with detailed supporting information about how the interested entity is able to meet the requirements outlined in this national coverage determination to the following address or via email to caginquiries@cms.hhs.gov.
Centers for Medicare & Medicaid Services
Center for Clinical Standards and Quality
Director, Coverage and Analysis Group
ATTN: Lung Cancer LDCT Screening
Mail Stop: S3-02-01
7500 Security Blvd.
Baltimore, MD 21244
Information regarding CMS-approved registries will be posted on the CMS website.
C. Nationally Non-Covered Indications
Unless specifically covered in this NCD, any other NCD, or in statute, preventive services are non-covered by Medicare.


